Introduction:
The use of medical devices has improved healthcare enormously. However, like any technology, medical devices also carry risks. These risks require vigilance. Post-marketing surveillance plays a key role in ensuring patient safety by monitoring devices after they are in clinical use. This article explores why post-marketing surveillance matters and how India is strengthening it through Medical Devices Regulations 2017 (MDR) and the Materiovigilance Programme (MvPI).
What is Post-Market Surveillance?
Post-market surveillance (PMS) refers to continuously monitoring the safety and efficacy of a medical device. This monitoring happens after the device enters the market and is in clinical use. It aims to promptly identify any issues or problems with the device so that appropriate corrective actions can be taken.
The Need for Post-Marketing Surveillance
When medical devices are tested during initial clinical trials, the sample sizes are small, and the duration is short compared to real-world long-term use. Hence, not all issues are identified prior to approval. Once the device is launched, it goes into varied patient populations and settings. Continuous PMS helps in picking up adverse events or device failures that happen at low frequencies or take time to manifest.
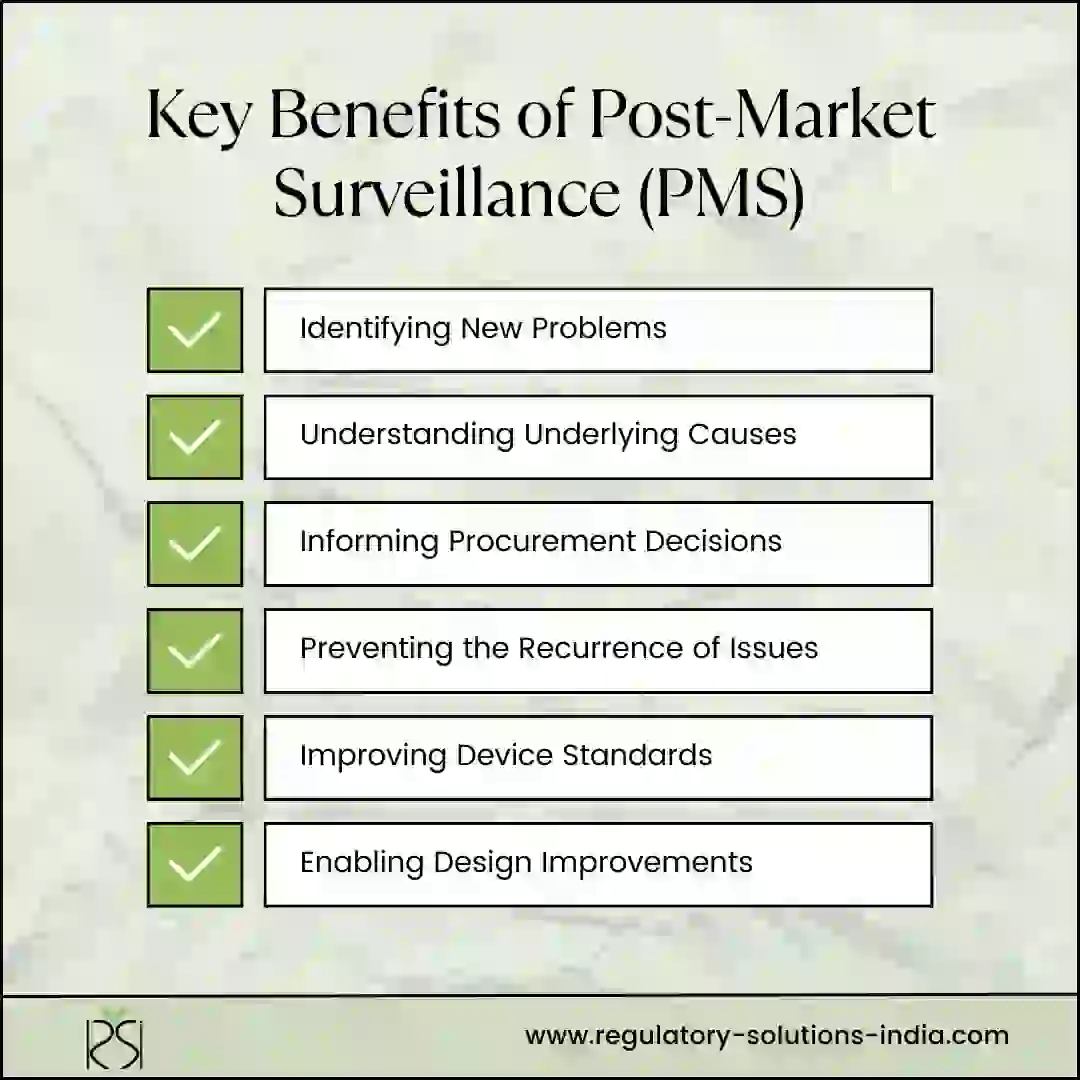
Some key benefits of medical device post-marketing surveillance are:
1. Identifying New Problems: Clinical trials have limited duration and patient sample size. Issues can emerge during long-term device use in diverse populations that pre-market trials cannot predict. Post-marketing surveillance allows proactively picking up adverse events not known earlier.
2. Understanding Underlying Causes: Investigating adverse events reveals failure modes and whether issues are device-related or due to use errors. Root cause analysis is key to finding solutions.
3. Informing Procurement Decisions: Systematically generated evidence on device safety performance enables making informed procurement choices. It prevents needless expenditure on devices with poor safety records.
4. Preventing the Recurrence of Issues: Analyzing event data helps identify recurrent problems. Alerts to hospitals can prevent similar adverse events. Manufacturers can implement corrections worldwide.
5. Improving Device Standards: Real-world data aids the revision of safety standards to address problematic device features or technology risks found post-marketing.
Enabling Design Improvements: Manufacturers can utilize surveillance insights to redesign products, improve materials and components, update software or strengthen cybersecurity.
Post-Market Surveillance: Requirements and Reporting
Post-market surveillance (PMS) ensures a medical device stays safe after being sold. The Medical Device Rules (MDR) 2017 outline PMS requirements and reporting in India. The important elements of Post-market Surveillance are as follows:
Complaint Handling System: Manufacturers/Local India agents must have a robust complaint handling system in place which includes Standard operating Procedures for handling complaints and preventive/corrective actions, etc.
Adverse Event (AE) and Serious Adverse Event (SAE): An Adverse Event (AE) is any event or other occurrence that reveals any defect in a medical device or concerns any adverse effect arising out of the use thereof. Whereas a serious adverse event means an untoward medical occurrence that leads to a death, or a serious deterioration in the health of the subject or foetal distress.
Field Safety Corrective Actions (FSCA): A field safety corrective action may include recalls, software upgrades, user notifications, added training etc. with an aim to reduce issues and ensure ongoing safety. In all such cases the manufacturers must promptly inform CDSCO of risks from devices that seem unsafe using the prescribed FSCA form for reporting.
Data Sources for PMS
1. Adverse event reports: Important safety data is gathered from adverse event reports associated with the use of the medical device. Manufacturers, healthcare providers, patients, and other stakeholders are required to submit reports of any adverse events or incidents. Analysis of these reports of problems or issues enables identifying risks and taking corrective actions to improve device safety. Adverse event reporting is a crucial component of medical device post-market surveillance.
2. Device registries: Device registries actively collect predefined clinical, technical and safety data on real-world use of a medical device type. This enables long-term monitoring of device performance and safety outcomes.
3. User complaints: Complaints reported by device users, healthcare providers, etc., are analyzed to identify potential issues with device performance or safety.
4. New clinical studies: Additional clinical studies conducted post-approval provide further evidence on safety and effectiveness in defined patient groups and settings.
5. Research publications: Ongoing review of published studies and reports helps identify emerging safety issues or risk factors associated with the device.
6. Returned faulty device analysis: Detailed analysis of returned faulty or failed devices helps determine the root causes and modes of failure.
7. Surveys, social media, sales data: Social media listening, market surveys, sales data analysis, etc., also provide supplementary data.
This data identifies risks, failure modes, patient impacts, and areas needing action. The goal is continuous monitoring to keep devices safe through collaborative work between manufacturers and regulators.
The Vital Role of Stakeholders
For post-marketing surveillance to work, a concerted effort is needed from all stakeholders:
– Healthcare professionals need to document and report any adverse events diligently.
– Hospitals must investigate failures and share data openly.
– Clinical/biomedical engineers are crucial for the technical investigation of incidents.
– Manufacturers need to undertake transparent root cause analysis.
– Regulators have to issue guidance and take data-driven actions.
– A culture of safety and ethics must underpin the system.
Investment in training, infrastructure, and efficient processes is required to realize the full benefits. Post-marketing surveillance is thus a collaborative endeavor for improving healthcare quality and outcomes.
The Materiovigilance Programme of India
Launched in 2015, MvPI aims to monitor device safety, guide policies and regulations, and promote safe use. The Indian Pharmacopoeia Commission is the National Coordination Center. Key aspects include:
– Monitoring adverse events and investigating signals
– Assessing device risks and causes of failures
– Communicating alerts and advisories
– Guiding regulatory controls and corrections
– Enhancing standards and manufacturer vigilance
MvPI has established vigilance monitoring centers throughout India and introduced an adverse event reporting form for gathering data from manufacturers, healthcare providers, and other stakeholders. Truly harnessing its potential requires active participation from all stakeholders.
Conclusion:
Post-marketing surveillance provides a valuable ongoing feedback loop for ensuring patient safety and optimizing medical device use. MvPI creates a robust surveillance infrastructure in India. But, realizing its potential requires the participation of all stakeholders. With a coordinated effort, post-marketing surveillance can enable proactive and preventive vigilance over the entire device life cycle.– Monitoring adverse events and investigating signals
– Assessing device risks and causes of failures
– Communicating alerts and advisories
– Guiding regulatory controls and corrections
– Enhancing standards and manufacturer vigilance
MvPI has established vigilance monitoring centers throughout India and introduced an adverse event reporting form for gathering data from manufacturers, healthcare providers, and other stakeholders. Truly harnessing its potential requires active participation from all stakeholders.
Contact Us for Your Regulatory Needs:
Regulatory Solutions India is here for all your regulatory needs, including post-market surveillance. Let us guide you through the complex world of regulations and ensure that your medical devices are safe and effective. Your success in healthcare is our top priority.
FAQ
1: What is post-market surveillance for medical devices?
Post-market surveillance (PMS) is the continuous monitoring of the safety and efficacy of medical devices after they have entered the market and are in clinical use. It aims to promptly identify any issues or problems with the devices.
2: Why is post-market surveillance necessary?
Post-market surveillance is necessary because clinical trials conducted before a device's approval have limitations, such as small sample sizes and short durations. PMS helps identify issues that may arise during long-term use in diverse patient populations.
3: How is post-market surveillance conducted in India?
Post-market surveillance in India is outlined in the Medical Device Rules (MDR) 2017. It involves Field Safety Corrective Actions (FSCA), reporting adverse events, data collection, recalling unsafe devices, and gathering data from various sources like adverse event reports and user complaints.
4: What is the role of stakeholders in post-market surveillance?
Stakeholders, including healthcare professionals, hospitals, clinical engineers, manufacturers, regulators, and others, play a vital role in documenting, reporting, investigating, and taking corrective actions related to adverse events. Their collaborative efforts ensure the effectiveness of PMS.