The Indian medical device market is advancing rapidly and is currently valued at approximately USD 12 billion, which is expected to grow significantly, reaching around USD 50 billion by 2030. This presents an ideal opportunity for manufacturers and importers to introduce their medical devices in India.
However, launching a medical device in India is a complex process that requires manufacturers to navigate stringent regulatory approval pathways. To successfully launch a product, it is essential to have a comprehensive regulatory strategy in place.
In this blog, we will guide you how a regulatory strategy report can help you in introducing a medical device in Indian market.
Before delving into a detailed regulatory strategy report, let’s first identify the governing body for the registration of medical devices in India and clarify who is eligible to apply for medical device registration.
Which is the governing body for medical device registration in India?
The Central Drugs Standard Control Organization (CDSCO) is the National Regulatory Authority of India for medical device registration in India.
Who can apply for medical device registration under CDSCO?
The parties that can register a medical device under CDSCO are as follows:
- The manufacturer who has a registered office in India.
- The authorized representative of the manufacturer.
- The manufacturer’s subsidiary.
- Importer.
- Domestic manufacturer.
For detailed information regarding CDSCO Medical Device Registration, you can read our blog “ 7 Key Steps In CDSCO Medical Device Registration: Easy Guide” .
What is a Regulatory Strategy Report?
A regulatory strategy report is a detailed document that outlines the plan and approach a company will take to navigate the regulatory approval process for the introduction of their medical devices in Indian market. This report is typically a roadmap for manufacturers and importers to ensure compliance with local laws, regulations, and standards for a successful market entry.
Why a Regulatory Strategy Report is Important for Manufacturers and Importers?
With a well-structured regulatory strategy report, manufacturers and importers can ensure timely market access, minimize regulatory risks, and maintain compliance with the evolving regulatory environment. In other words, an effective regulatory strategy report enables manufacturers and importers to plan ahead and allocate resources efficiently, ensuring the successful launch of their medical device in the Indian market.
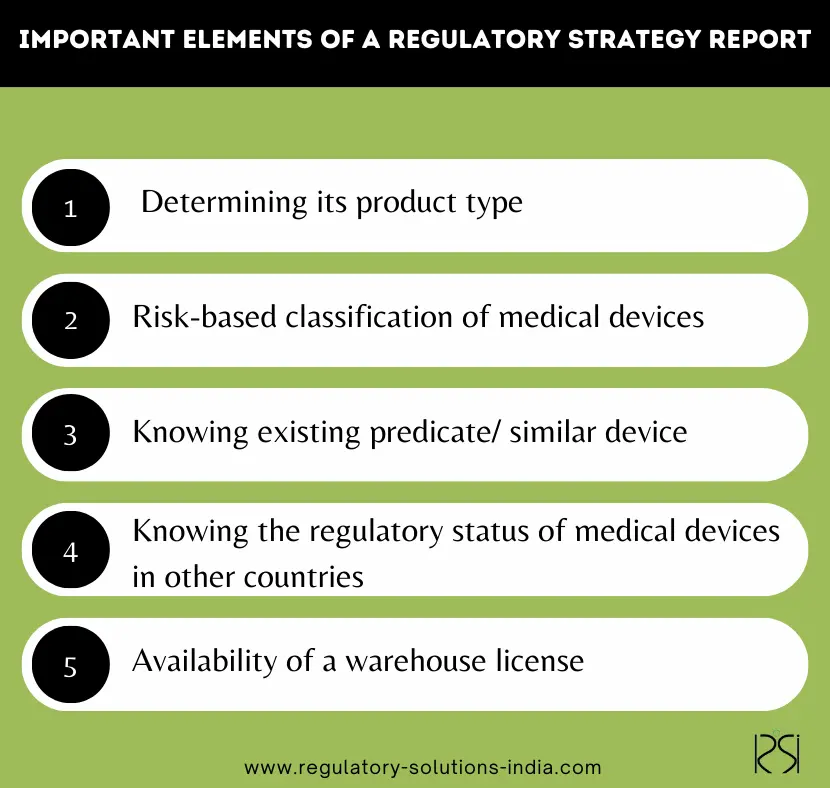
What are the Important elements of a Regulatory Strategy Report?
A systematically designed regulatory strategy report consists of several key elements that help in determining the regulatory pathway for a device introduction in India. These elements are as follows:
- Product Type: The first and foremost step in introducing a medical device is determining the product type, as each type is governed by distinct regulatory frameworks. The primary product types include Medical Devices, In Vitro Diagnostics (IVDs), Cosmetics, and Drugs.
- Risk Classification: Establishing the risk class of the medical devices is a critical step in the regulatory process, as it directly impacts the approval requirements and regulatory pathway.
- Existing Predicate / similar device in India: In India, an existing predicate/ similar device helps demonstrate that the proposed device is substantially equivalent to a legally approved one. However, if you have a medical device without a predicate, the regulatory approval pathway would involve additional steps. For more details on such devices and how to get them registered read our blog “ CDSCO Approval Process For Medical Devices Without Predicate In India”.
- The regulatory status of the device in other countries: Knowing the regulatory status of medical devices in other countries serves not only as evidence of a device’s safety, efficacy, and quality but may help qualify for certain waivers e.g. for clinical trials.
- Warehouse Availability & Registration: The availability of a warehouse and a registration certificate (in Form MD-42) is a mandatory pre-requisite for introduction of a medical device in India. For more details on this do refer to our blog “Registration Certificate for Sale”.
Conclusion
Introducing a medical device into India’s rapidly growing market presents business opportunities as well as regulatory complexities for manufacturers and importers. By having a customized regulatory strategy report for your product portfolio, you can have a step-by-step guide to determine the regulatory pathway for a device introduction in India.
Additionally, the support from an expert partner proves invaluable for compliance and commercial success. Regulatory Solutions India (RSI) offers 12+ years of experience in successfully registering medical devices, IVDS, cosmetics and drugs.
Contact Us today. RSI can guide your company through India’s device approval process smoothly. Partnering with RSI can help you bring your medical innovations to patients in India’s fast-growing healthcare market.