The Indian medical device market is growing rapidly, and the current medical device market size is estimated at $11 billion and is expected to reach $50 billion by 2025. This presents an opportunity for manufacturers and importers to launch their medical devices in India.
The Regulatory approval pathway for a Medical Device without predicate can have variations and hence can be a bit complex to navigate through. In this blog, we will guide you through the overall process to get approvals for such devices.
What is a Medical Device without predicate?
Let us first understand what is meant by a predicate device.
The Medical Device Rules define a predicate as “a device, first time and first of its kind, approved for manufacture for sale or for import by the Central Licensing Authority and has the similar intended use, material of construction, and design characteristics as the device which is proposed for licence in India”
Thus a Medical device without predicate will be one which does not have a similar device as defined above in the Medical Device Rules 2017.
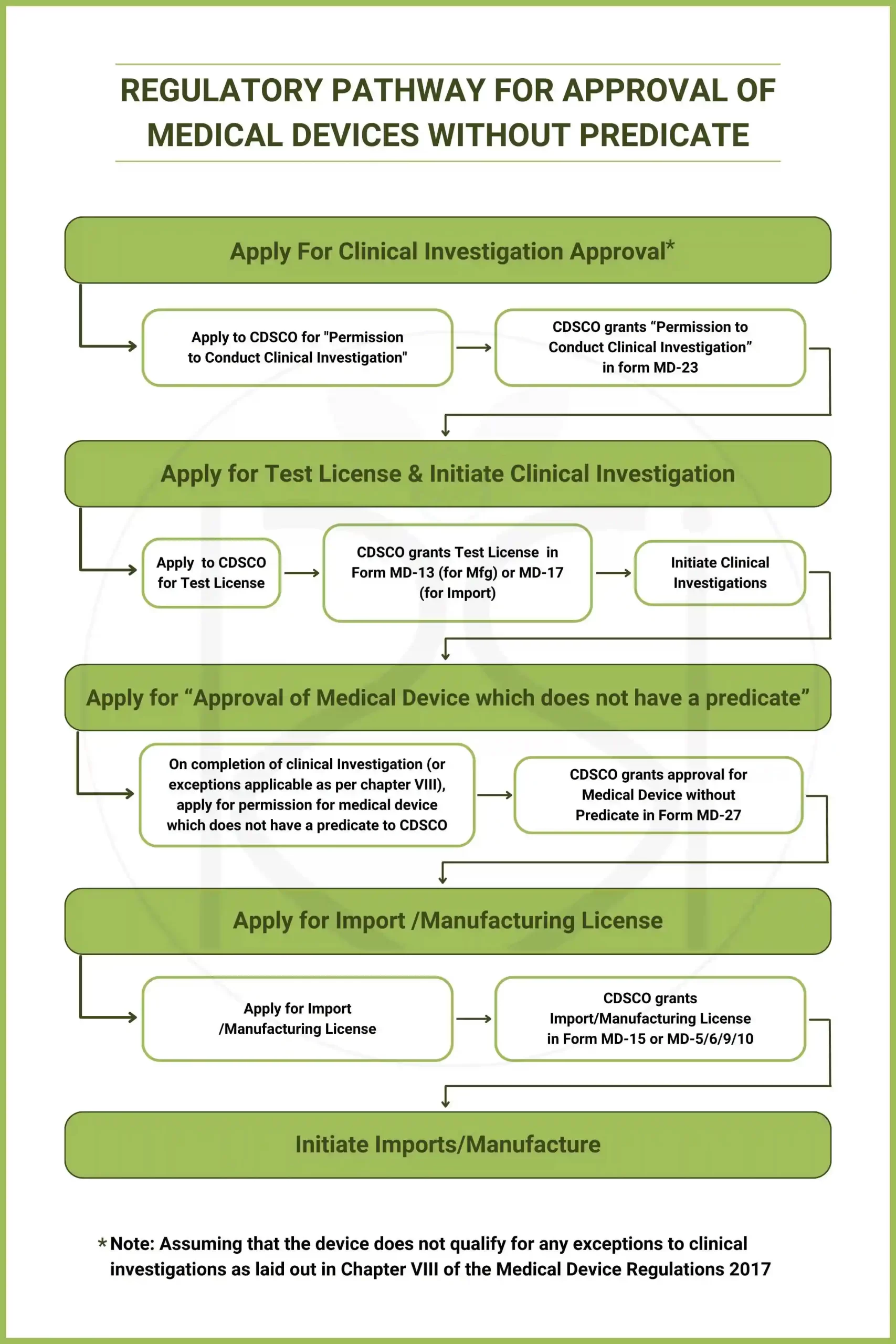
Regulatory Pathway for Approval of Medical Devices without predicate:
1. Clinical Investigation: Manufacturers/Importers must conduct clinical investigation to establish safety and efficacy as per rules set by India’s Central Drugs Standard Control Organization (CDSCO), specifically under Chapter VII of the Medical Device Rules 2017. Application must be made in Form MD-22 and approval is granted in MD-23. There are some exceptions to clinical investigation requirements by CDSCO which we will discuss below.
2. Clinical Investigation exceptions for Class B, C & D: Chapter VIII of the MDR 2017 specifies that, clinical investigation may not be required to be submitted where
- the medical device without predicate is approved by the regulatory authorities of either the United Kingdom or the United States of America or Australia or Canada or Japan and
- the said device has been marketed for at least two years in that country and
- the Central Licensing Authority is satisfied with the data of safety, performance, and pharmacovigilance of the device and
- there is no evidence of any difference in the behavior and performance in Indian population.
On compliance to the above conditions and a written undertaking by the applicant to conduct post marketing clinical investigation, as per protocol approved by the Central Licensing Authority, a clinical investigation may not be required prior to approval. There are a few additional exceptional situations prescribed therein e.g. national emergencies, epidemic etc. wherein the clinical data requirements may be abbreviated, deferred or omitted.
3. Test License & Clinical Investigation start: Obtain test License in MD-13 (for manufacturing) or MD-17 (for import) and conduct clinical investigation.
4. Apply for “Approval of Medical Device which does not have a predicate” (in Form-26) and Obtain Permission in Form MD-27 which would be subject to conditions as outlined in Chapter VIII of the MDR 2017.
5. Apply for Import/Manufacturing License: Subsequent to the Form MD-27, manufacturers/importers can apply for the Import License (in Form MD-14/ MD-3/7), submitting necessary documents.
6. Initiate Imports/Manufacture : Upon receiving the Import / Manufacturing License the import/manufacturing of the “medical device without predicate” can be initiated however at all times complying to the clinical investigation and other prescribed requirements specifically those in Chapter VII and VIII of the MDR 2017.
How to Get Permission for Conducting Clinical Trials:
1. Identify an Indian Clinical Research Organization (CRO): Choose an Indian CRO to manage and oversee clinical trials.
2. Submit Form MD-22 for Clinical Trial Permission: Prepare and submit Form MD-22 with supporting documents to CDSCO, requesting permission to conduct clinical trials.
3. Grant of ‘Permission to Conduct Trials’ (in Form MD-23): Upon successful review, CDSCO grants ‘Permission to Conduct Trials’ in Form MD-23.
4. Commence Clinical Trials After Ethics Committee Approval & CTRI Registration: Following Ethics Committee approval and Clinical Trials Registry – India (CTRI) registration, commence the clinical trials.
Conclusion:
Introducing a medical device not having predicate in India’s thriving market presents immense opportunities along with regulatory complexities. By following the step-by-step approval roadmap and securing import permissions, manufacturers/importers can realize significant benefits.
Additionally, support from an expert partner proves invaluable for compliance and commercial success. Regulatory Solutions India (RSI) offers 12+ years of experience in successfully registering healthcare products in India.
Contact Us today. RSI can guide your company through India’s device approval process smoothly. Partnering with RSI helps bring your medical innovations to patients in India’s fast-growing healthcare market.
FAQ
What are the key requirements to introduce medical device without predicate in India?
The device having no comparable product i.e. predicate registered in India should undergo clinical trials for safety/efficacy, and obtain permissions in Form MD-27 by submitting the application in Form MD-26 along with supporting documents. Finally obtain the import /manufacturing license in Form MD-15 /MD-5 or MD-8.
Who can apply to manufacture or import an Investigational medical device in India?
Indian manufacturer or authorized agent of foreign manufacturer can apply to manufacture/import investigational medical devices in India.
What is Form MD-26?
Form MD-26 is the application that must be submitted to request permission to import or manufacture a medical device not having predicate in India.
What is Form MD-27?
Form MD-27 is the approval granted by India's Central Drugs Standard Control Organization (CDSCO) if the Form MD-26 application is found satisfactory.
What fees are required to obtain permission in form MD-27?
The application fee is INR 50,000 to import or manufacture a medical device without predicate in India as per current regulations. Additional fees would be applicable for the clinical trials and the import license.