The Central Drug Standard Control Organization (CDSCO) has recently made a change to how safety reports for medical devices and in-vitro diagnostic devices are submitted in India. Starting on March 19, 2024, all manufacturers have to submit these reports online through the Sugam portal. This new method is meant to make things easier and more organized. In this blog, we’ll talk about why these reports are important, what they should include, and how to submit them.
What is a Periodic Safety Update Report (PSUR)?
Periodic Safety Update Reports (PSURs) are crucial reports, applicable to investigational medical devices, (i.e. devices without predicate) that manufacturers/importers of such medical devices need to submit to demonstrate how their products are performing after they have been sold. These reports are a key part of Post-Market Surveillance (PMS), a practice that monitors the safety and performance of investigational medical devices on the market.
What should be included in PSUR?
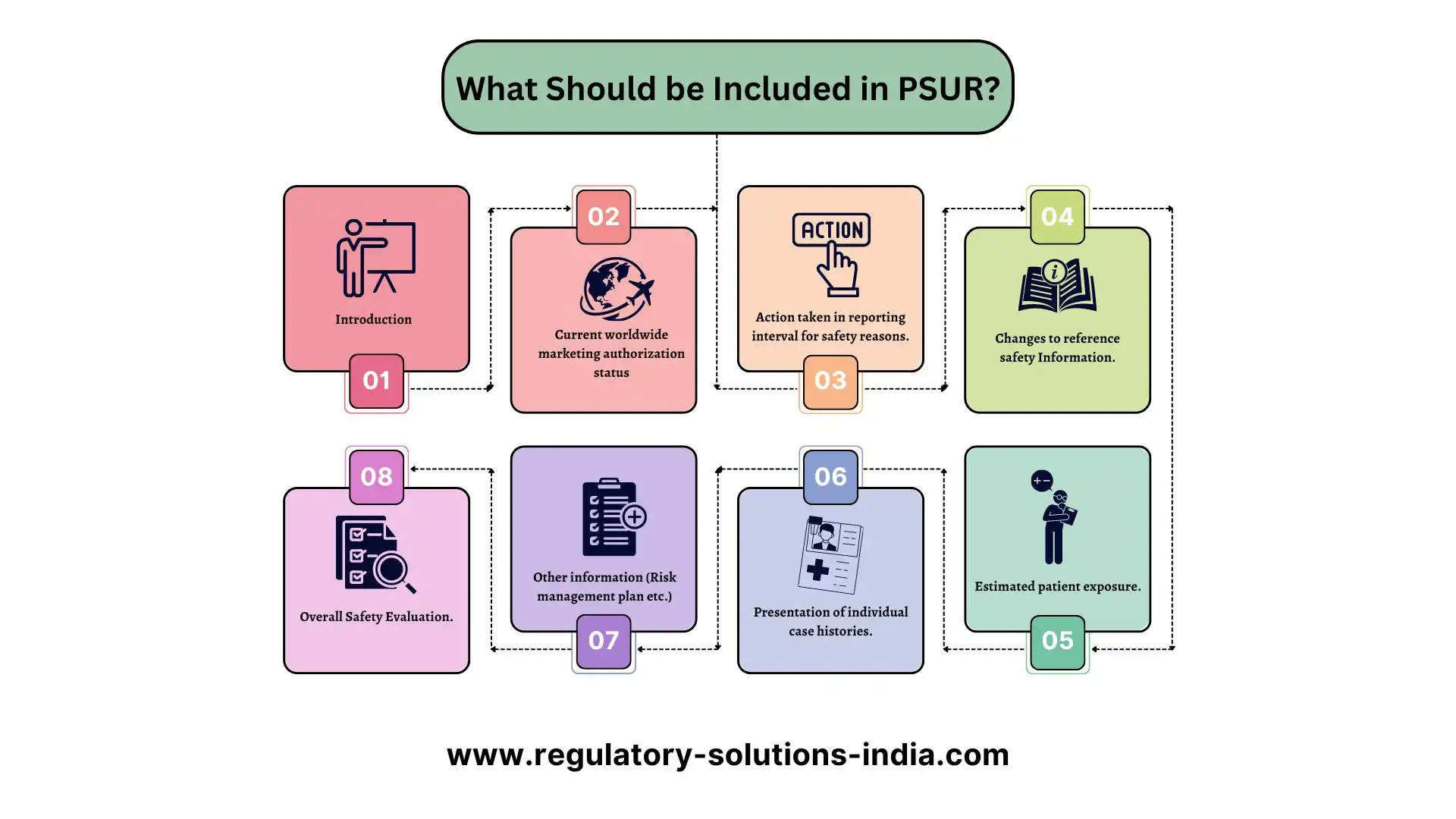
A PSUR should have several parts that give a full picture of how a medical device is doing. Broadly, the MDR 2017 recommends that a PSUR to be structured as follows:
- Introduction: Complete with the device details (intended use, mode of operation, etc.) , the reporting interval and description of the intended population.
- Current worldwide marketing authorization status: Details of countries, where the device is approved, and/or countries where it is withdrawn with reasons thereof.
- Action taken in reporting interval for safety reasons: Description of any significant safety actions during the reporting period.
- Changes to reference safety Information: Any significant changes to reference safety information.
- Estimated patient exposure: Estimates of size and nature of the population using the device, and detailed case histories of any problems people have had with it.
- Presentation of individual case histories: Individual case information available to a license holder including brief case narrative, medical history indication, causality assessment etc.
- Studies: Summaries of new safety findings that may affect device safety information.
- Other Information: This should include details about the risk management plan.
- Overall Safety Evaluation: Summary of the safety concerns, benefit evaluation and Risk-benefit analysis along with the safety profile of the medical device.
Who Needs to Submit PSURs?
The companies that manufacture/import the investigational medical devices are the ones who need to prepare and send these reports. It’s important they do a good job of making sure the reports are accurate and sent on time.
When and How to Submit a PSUR
Here’s when and how to send these reports:
- For the First Two Years: A report needs to be sent every six months.
- For the Next Two Years: After that, a report is needed once a year.
- Longer If Needed: Sometimes, more reports might be needed based on health needs.
- All reports should now be sent online through the Sugam portal, which helps keep everything in order and easy to manage.
Conclusion:
Periodic Safety Update Reports are more than just paperwork—they’re crucial for keeping investigational medical devices safe after they are sold. By carefully looking at safety information, companies and regulators can make decisions that keep everyone safe and ensure devices work as they should. This ongoing monitoring is key to keeping trust in medical technology high.
At Regulatory Solutions India (RSI), we specialize in providing regulatory consultancy services for medical devices If you need help navigating the submission process or ensuring compliance with the latest regulations, contact us.
FAQ
1. What is a PSUR, and why is it important for medical device manufacturers?
A Periodic Safety Update Report (PSUR) is a crucial report that investigational; medical device importers/manufacturers submit to demonstrate how their products are performing after they’ve been sold. It’s essential because it helps monitor the safety and performance of devices post-market, ensuring they remain safe and effective for patient use.
2. What information should be included in a PSUR?
A PSUR should include details such as the device’s intended use, where it’s approved for sale, any safety actions taken, updates to safety information, estimates of device usage, research findings, and an overall safety review.
3. Who is responsible for submitting PSURs, and when should they be submitted?
The responsibility for submitting PSURs lies with the companies that manufacture/import such investigational medical devices. PSURs should be submitted every six months for the first two years post-marketing, then annually for the next two years. Additional submissions may be required based on health needs.
4. How has the submission process for PSURs changed in India?
As of March 19, 2024, the Central Drug Standard Control Organization (CDSCO) has mandated that all manufacturers/importers submit PSURs online through the Sugam portal. This change aims to streamline the submission process and ensure greater organization.
5. Why are PSURs considered crucial for maintaining trust in medical technology?
PSURs are more than just paperwork—they play a critical role in keeping investigational medical devices safe after they're sold. By carefully monitoring safety information and addressing any issues promptly, companies and regulators can uphold the trust of patients and healthcare professionals in medical technology.