The European Union (EU) is one of the largest and most lucrative medical device markets in the world. While it offers significant opportunities for Indian manufacturers, it is also one of the most highly regulated, making market entry both rewarding and challenging. Selling medical devices in the EU requires more than just product quality; it demands strict compliance with complex regulatory standards.
In this blog, we’ve outlined the essential steps Indian manufacturers need to take to enter the EU medical device market and explained how you can simplify your journey. Continue reading to explore the process in detail.
Why Indian Medical Device Manufacturers Should Explore the EU Market
The EU’s unified market spans 27 countries, giving manufacturers access to nearly 449 million people. With a growing demand for cost-effective and innovative medical devices, the EU presents a highly favorable opportunity for Indian manufacturers. However, to ensure patient safety and product quality, the region enforces stringent regulations, including the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
What is the EU MDR?
The Medical Device Regulation (2017/745/EU) is a comprehensive set of rules that govern the manufacture and distribution of medical devices within the European Union. These regulations are designed to ensure that all devices meet high safety, performance, and quality standards, ensuring they are safe and effective for use.
Who Is Required to Comply with the EU MDR?
Any medical device manufacturer intending to sell products in the European Union must comply with the EU Medical Device Regulation (MDR). This requirement applies to both new and existing devices. Even if a device was previously certified under the Medical Device Directive (MDD), it must now meet the updated standards outlined in the EU MDR.
To demonstrate compliance, the device must obtain CE marking before it can be legally distributed within the EU market.
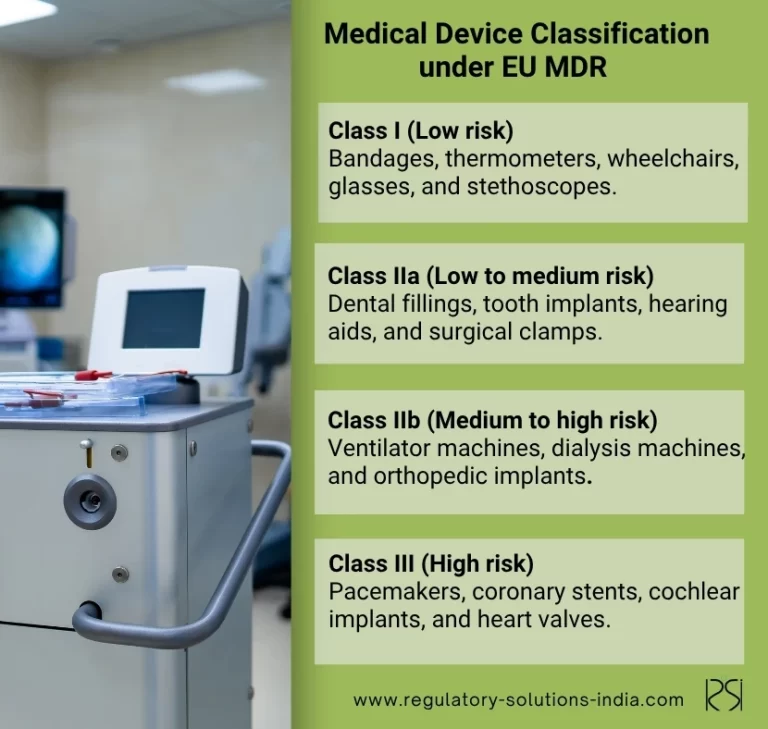
Medical Device Classification under EU MDR
Under EU MDR, medical devices are classified based on their risk to patient safety. They are as follows:
- Class I-Low Risk
Class I includes the lowest risk medical devices and can be further divided into:
- Class I – Standard low-risk devices (non-sterile, non-measuring, non-reusable surgical devices)
- Class Is – Sterile devices
- Class Im – Measuring function devices
- Class Ir – Reusable surgical instruments
Examples of EU MDR Class I medical devices are bandages, thermometers, wheelchairs, glasses, hospital beds, and stethoscopes. Most medical devices in Class I can be self-certified
- Class IIa- Low-to-Medium Risk
Class IIa includes invasive medical devices that are intended for short-term use.
Examples are dental fillings, tooth implants, hearing aids, diagnostic ultrasound equipment, and surgical clamps.
- Class IIb- Medium-to-High Risk
Class IIb includes invasive medical devices that are intended for long-term use.
Examples include ventilator machines, dialysis machines, and orthopedic implants.
- Class III-High Risk
Class III includes implantable medical devices intended for long-term use.
Examples are pacemakers, coronary stents, cochlear implants, and heart valves.
A Roadmap for Indian Medical Device Manufacturers to Enter the EU Market
Before selling medical devices in the EU market, manufacturers must comply with the Medical Device Regulation (MDR), which outlines a series of mandatory steps. To support you in this journey, we’ve outlined the key steps that can help you navigate the compliance process smoothly and efficiently.
- Step 1: Classify Your Medical Device
The first step is to determine the classification of your medical device. Based on the level of risk it poses to patients, devices are categorized into one of the following classes: Class I, IIa, IIb, or III. The classification determines the regulatory pathway, documentation requirements, and the level of Notified Body involvement needed for certification.
- Step 2: Establish a Robust Quality Management System (QMS)
Manufacturers are required to implement and maintain a Quality Management System (QMS) that is compliant with Article 10(9) of the MDR. For high-risk devices, the QMS must be certified by a Notified Body.
- Step 3: Prepare Technical Documentation
Preparing detailed technical documentation which is compliant with the MDR’s General Safety and Performance Requirements (GSPR) is mandatory. This includes device description, information on design, manufacturing, risk analysis, clinical evaluation, labeling, and instructions for use (IFU) and Post-Market Surveillance Documents - Step 4: Appoint an EU Authorised Representative (AR)
Non-EU manufacturers, including Indian manufacturers, must appoint an EU-based Authorized Representative (AR). This AR serves as the local contact for EU national Competent Authorities, facilitates compliance, and must be identified on the product label and Declaration of Conformity (DoC). - Step 5: Apply for Conformity Assessment with a Notified Body
This step is only a requirement for higher class devices and Class I devices with a measuring function, sterile condition, and reusable. For CE marking of these higher-class devices, you will need to apply to a Notified Body of your choice for conformity assessment of your device.
For Class I (self-certified) devices, there is no Notified Body intervention.
The selected notified body will perform the audit of your QMS and manufacturing facility and review your technical documentation.
If the NB finds deficiencies, the manufacturer must correct non-conformities and provide evidence of compliance.
Once compliance has been demonstrated, the CE Certificate will be awarded
- Step 6: Register in EUDAMED
Register your device and company details in the European Database on Medical Devices (EUDAMED). This ensures traceability and transparency for regulators and consumers.
- Step 7: Affix CE Marking to Device
When all the previous steps have been completed, you can affix the CE Marking. For simple Class I devices, the CE Mark must be as shown below.
For Class Im, ls, lr , or higher class devices (Class IIa, IIb and Class III) the CE Mark must include the notified body number, as shown below.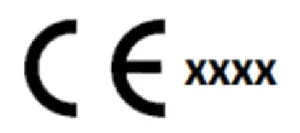
- Step 8: Post Market Surveillance (PMS)
Manufacturers must continuously monitor the device’s safety and performance after it is placed on the market. This includes reporting adverse events and incidents to the appropriate regulatory authorities.
By following this roadmap, Indian medical device manufacturers can confidently expand into the EU market while meeting all compliance requirements. Partnering with an experienced EU Authorised Representative and regulatory consultant can make the process smoother and more efficient.
How Can Regulatory Solutions India Help You?
Regulatory Solutions India (RSI), established in 2011, offers EU Authorised Representative services through our partners in EU to assist non-EU manufacturers, particularly those based in India, in complying with EU MDR requirements. The EU authorized Representative will ensure that your documentation complies with MDR standards while facilitating seamless CE marking and market entry.
Partner with RSI for reliable, efficient access to the European medical device market.