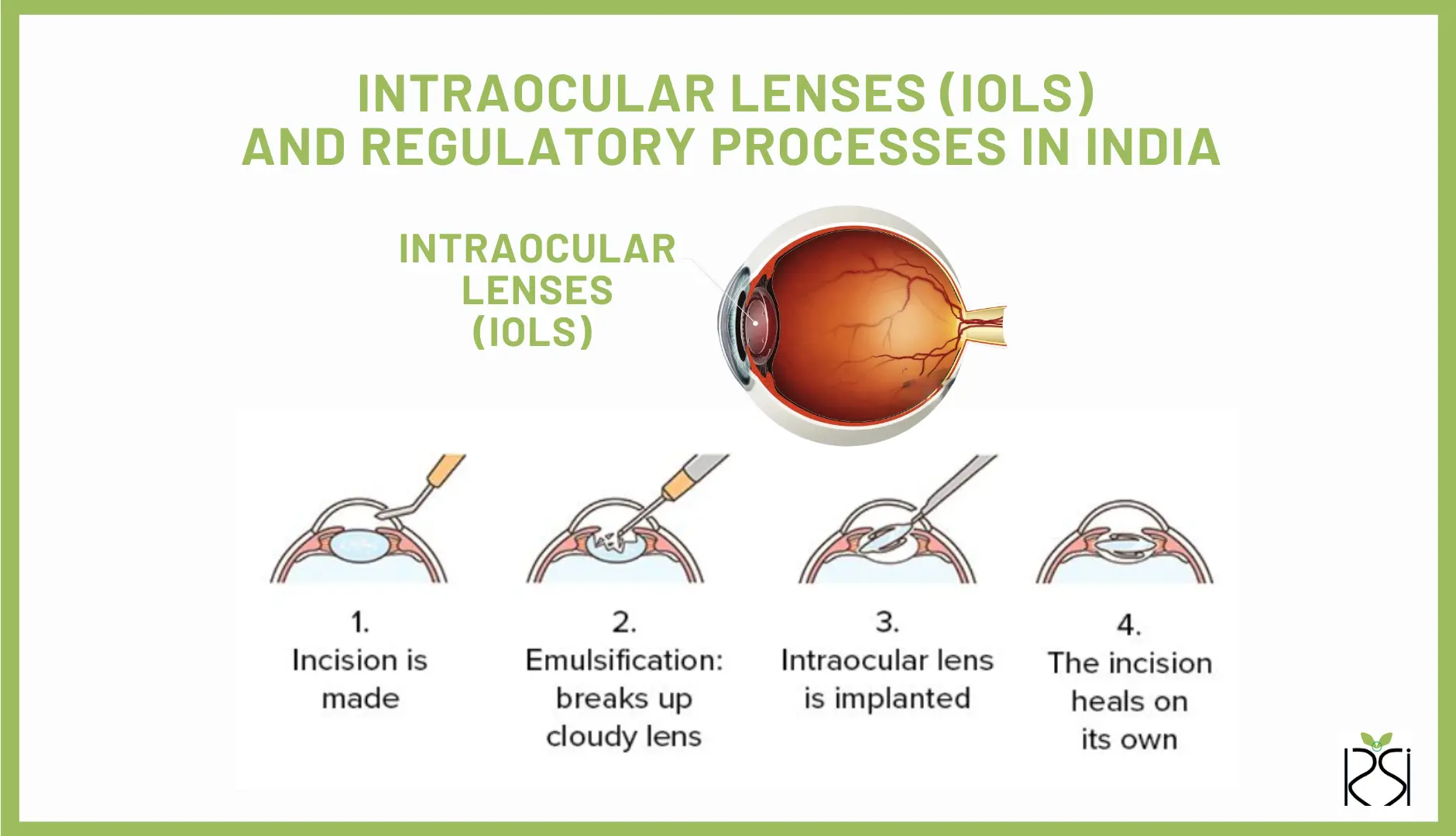
Intraocular Lenses (IOLs) are special lenses put inside the eye during surgery to replace the natural lens. This often happens when someone has cataract surgery, but they can also be used for other vision correction surgeries.
What Are Intraocular Lenses (IOLs) Regulatory Processes?
IOLs are artificial lenses made from clear plastic, silicone, or acrylic. They help people see better by focusing light onto the retina, just like the natural lens in our eyes. Unlike contact lenses that sit on the eye’s surface, IOLs are implanted inside the eye.
Types of Intraocular Lenses
There are different types of IOLs for different vision needs:
1. Monofocal IOLs: These lenses have one focusing distance, usually for clear distance vision. You might still need glasses for reading or close-up work.
2. Multifocal IOLs: These lenses provide multiple focal points, so you can see clearly at different distances (near, intermediate, and far). This reduces the need for glasses.
3. Toric IOLs: These lenses help people with astigmatism, which is when the cornea is curved unevenly.
4. Accommodative IOLs: These lenses move or change shape inside the eye, offering a more natural range of vision.
5. Extended Depth of Focus (EDOF) IOLs: These lenses provide a continuous range of vision with one elongated focal point, offering better intermediate vision without causing glare or halos.
Uses of Intraocular Lenses
IOLs are used in several situations:
● Cataract Surgery: The most common use of IOLs is to replace the natural lens when it becomes cloudy due to cataracts.
● Refractive Lens Exchange (RLE): This surgery is similar to cataract surgery but is done to correct vision problems like near-sightedness, far-sightedness, and presbyopia.
● Phakic Intraocular Lens (PIOL): These lenses are implanted without removing the natural lens to correct high levels of near-sightedness or far-sightedness.
In India, the Central Drugs Standard Control Organization (CDSCO) regulates IOLs. Here’s what you need to know about the approval process:
Regulatory Approval for Intraocular Lenses in India
In India, the Central Drugs Standard Control Organization (CDSCO) regulates IOLs. Here’s what you need to know about the approval process:
Classification of Intraocular Lenses
As per the risk-based classification of medical devices under the Medical Device Rules (MDR), IOLs are classified under Category C, which means they are considered moderate-to-high risk devices.
Regulatory Pathway
● CDSCO Registration: All IOLs must be registered with the CDSCO. This includes submitting clinical data, technical details, and manufacturing information.
● Licensing: Manufacturers and importers need licences to manufacture or import IOLs.
● Clinical Evaluation: Clinical trials might be needed to prove that IOLs are safe and effective.
● Quality Management Systems: Compliance with standards like ISO 13485 is necessary to ensure safety and quality.
How RSI Helps in the Regulatory Process?
Regulatory Solutions India (RSI) is a leading regulatory consultancy in India that assists companies with medical device regulations. Our team comprises experts experienced in the regulatory requirements for medical devices, including IOLs. Here’s how we can help:
- Preparing and submitting regulatory documents
- CDSCO Registration/Licensing
- Submission of IL renewal/retention applications
- Post-market surveillance and compliance
Conclusion:
Understanding the rules and steps for getting approval for intraocular lenses in India can be challenging, but with the right help, it becomes easier. Knowing about the regulatory requirements is key for successful market entry. RSI has helped several companies over the years register their products over a wide range of applications, some of which are Ophthalmic, Neurological, General Hospital, Respiratory, Dental, Ear, Nose and Throat (ENT), Obstetrical and Gynecological (OG), Software, Operation Theatre (OT), Gastroenterological, Oncology and more.
By working with Regulatory Solutions India (RSI), you can ensure compliance and focus on bringing your IOL’s and other medical devices to market.