Introduction :
In the dynamic world of medical devices, regulations play a critical role in ensuring patient safety and product efficacy. The Government of India, recognizing the need to streamline the licensing process for medical devices, introduced the concept of “medical device grouping” as part of the Medical Device Rule, 2017 (MDR 2017). The primary aim of this concept is to simplify the process of applying for a license, making it faster and more cost-effective. To shed light on this important aspect of medical device regulations, this blog will explore what medical device grouping is and the various types of groupings as outlined by the Ministry of Health and Family Welfare (MoHFW) through its guidelines released-on March 16, 2018 and subsequent FAQ’s published on this important topic.
What is Medical Device Grouping?
Medical device grouping involves categorizing medical devices based on shared purposes or similar technology. This allows applicants to combine devices with the same intended use or common technology and apply for a single license for import or manufacturing. This not only simplifies the license application process for manufacturers and distributors but may also help reduce the applicable government fees depending on the grouping type, as explained in the next section.
Types of Medical Device Grouping:
The guidelines provided by the MoHFW offer clarity on how medical devices can be categorized and grouped for the license application. Here are the different types of grouping and their significance:
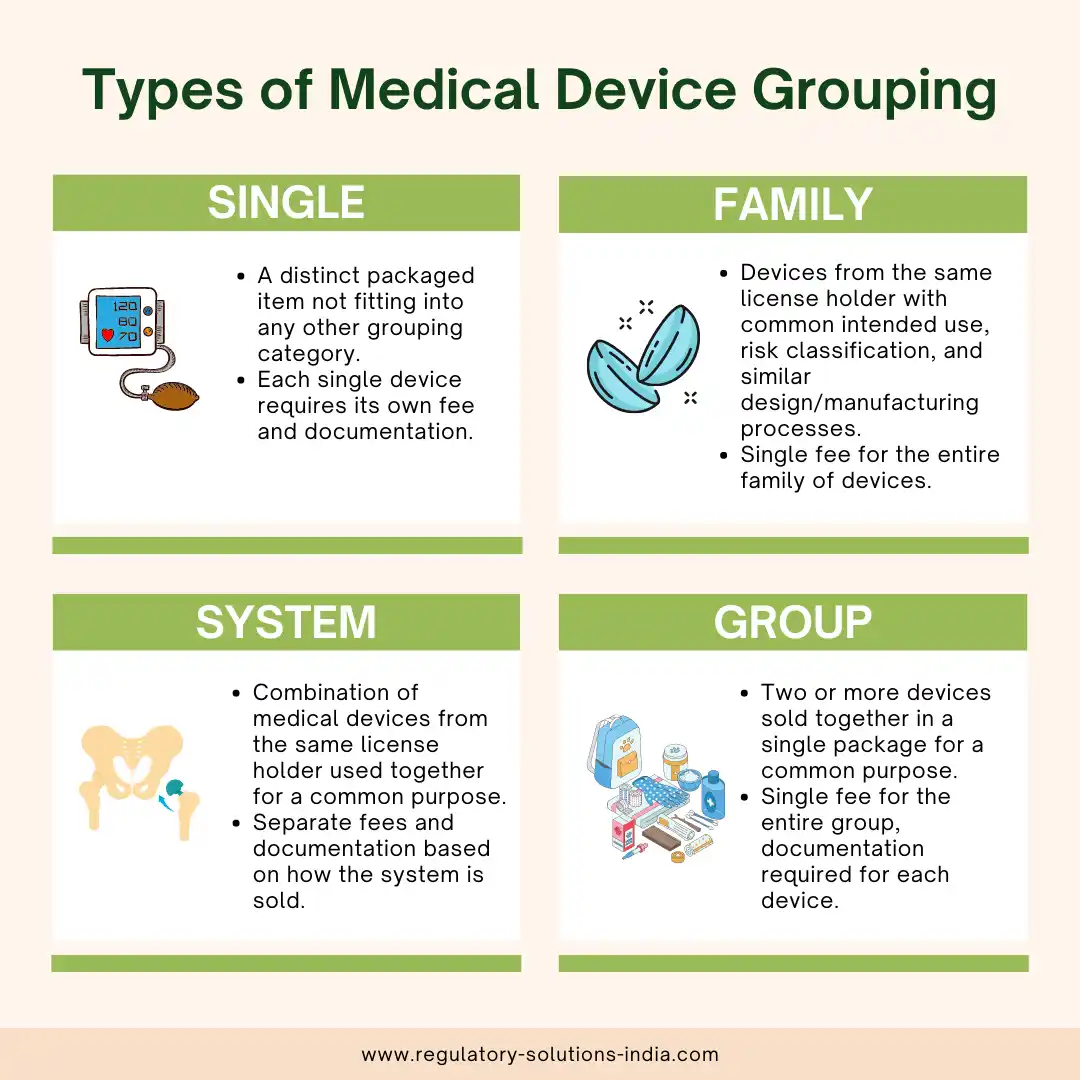
1. Single:
A single medical device refers to a distinct packaged item that does not fit into any other grouping category, such as families, systems, or group. Each single device requires its own separate fee and documentation. An example of this would be a company selling one single type of BP monitoring machine.
2. Family:
A medical device family includes devices from the same license holder with the same risk classification, common intended use, and similar design and manufacturing processes provided they have variations within the permissible scope. The family is licensed as a whole, and there is a single fee for the entire group. For instance, contact lenses with added UV protection can be grouped as a family since the additional feature does not alter the fundamental design and manufacturing process.
3. System:
A system refers to a combination of medical devices from the same license holder, intended to be used together for a common purpose.
If components of the system are used in more than one system, then they should be included in the application for each of those systems. Separate fees and documentation may apply based on how the system is sold. For example, a hip replacement system comprising femoral and acetabular components can be licensed as a system.
4. Group:
A medical device group consists of two or more devices sold together in a single package by the same license holder, serving a common purpose. The devices in the group may have different names, intended purposes and manufacturers. If a device in a group is used in another group, it should be included in the application for that other group. An example of this would be a first aid kit comprising medical devices such as bandages, gauzes, drapes, and thermometers, assembled together as one package for a common medical purpose by a product owner.
For a group of medical devices, a single fee is applicable (for the highest risk class product in the Group), and documentation is required for each device in the group.
Conclusion:
The introduction of medical device grouping is a commendable initiative by the Ministry of Health and Family Welfare (MoHFW), significantly enhancing the application process for medical device licenses. It not only simplifies and expedites the licensing process, but also makes it more cost effective for the license applicant. For further information on medical device grouping and regulatory matters, feel free to contact Regulatory Solutions India. For a group of medical devices, a single fee is applicable (for the highest risk class product in the Group), and documentation is required for each device in the group.
FAQ
1. What is medical device grouping?
Medical device grouping involves categorizing devices based on shared purposes or technology. It allows applicants to apply for a single license for devices with similar use or technology, simplifying the application process while making it cost-effective for the license applicant.
2. How are medical devices grouped?
Devices can be grouped as “Single," “Family," “System," or “Group." Each category has specific criteria and implications for licensing.
3. What is the difference between a Family and a Group?
A Family includes devices with the same risk classification, intended use, and manufacturing process, licensed together. In contrast, a Group may comprise multiple devices from different manufacturers sold together for a common purpose, even if they have different names and intended uses.
4. How can medical device grouping benefit manufacturers?
Medical device grouping simplifies the license application process, reducing costs as multiple devices can be grouped into one (when possible) and reduces application preparation time for importers/ manufacturers.