In India, the manufacture, import, and distribution of medical devices are regulated by the Central Drugs Standards Control Organization (CDSCO) under the Medical Device Rules 2017 to ensure the safety and efficacy of the devices used in the country. Manufacturers and Importers must obtain necessary approvals to manufacture, import, and distribute medical devices. These approvals ensure that medical devices are safe for public use and compliant with Indian regulations.
In addition to the above regular approvals there are also some specific approvals required by companies from time to time. One such approval is the Non-Conviction Certificate (NCC) issued by the central licensing authority CDSCO.
In this blog, we will discuss what a Non-Conviction Certificate is, its purpose, eligibility, and how to obtain it.
What is a Non-Conviction Certificate (NCC)?
A Non-Conviction Certificate (NCC) is a document issued by the Central Drugs Standard Control Organization (CDSCO) to medical device manufacturers and importers in India. It verifies that a company has not been convicted of any offenses related to serious injuries or deaths associated with its medical devices, reported malfunctions of its devices, or instances where its devices do not meet quality standards.
This certificate is often required by companies while bidding for tenders or requesting for procurements as it serves as evidence of a company’s regulatory compliance record.
Why is a Non-Conviction Certificate (NCC) Important?
A NCC demonstrates that a company is committed to regulatory compliance and product quality. It can significantly help manufacturers and importers in:
- Demonstrating Quality: NCC helps demonstrate that the entity adheres to ethical practices and has no legal liabilities relating to the products’ safety and efficacy.
- Requirements for Tenders and Procurements: In many tenders and requests for procurements, NCC is often required as the pre-qualification document as evidence of a company’s regulatory compliance record.
- Market Reputation: NCC, being a certificate issued by the central/state licensing authority, it helps a company showcase its market reputation, ensuring that clients can rely on it.
Who Is Eligible to Apply for a Non-Conviction Certificate?
A company that deals in medical devices can apply for the NCC in case it:
- Holds a valid manufacturing license that has been issued by the Central Licensing Authority (CLA) or State Licensing Authority (SLA) in India.
- Has no past record of conviction for violations under the Drugs and Cosmetics Act 1940 and its Rules.
Documents Required for Obtaining a Non-Conviction Certificate
Requirements for obtaining a Non-Conviction Certification are mentioned below:
- Valid manufacturing/import license.
- List of products for which the company is requesting NCC.
- Legal Undertaking from the manufacturer or importer stating that no action has been initiated against them.
Who will issue a Non-Conviction Certificate?
The licensing authority (CLA or SLA) that issued your manufacturing/import license will issue the NCC.
What is the validity of a Non-Conviction Certificate?
NCC is typically valid for one year from the date of its issuance or until the expiry of a company’s manufacturing license (whichever is earlier).
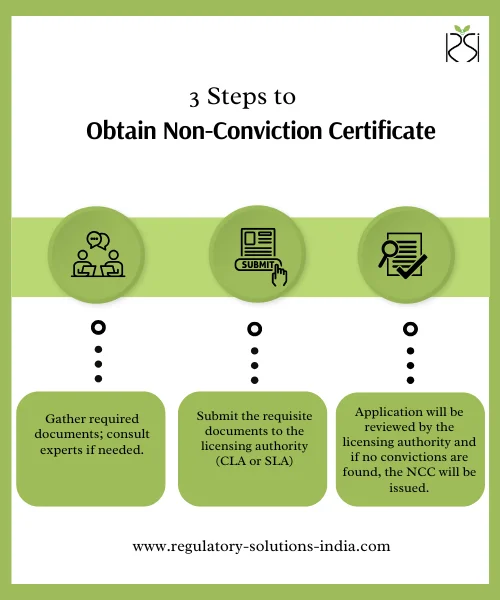
How to Obtain Non-Conviction Certificate
- Prepare and collate the necessary documents required as indicated in the section above. If necessary, you may take assistance from regulatory consultants with experience in this field.
- Submit the online application with the requisite documents to the licensing authority (CLA or SLA) requesting the issuance of a Non-Conviction Certificate.
- The licensing authority will review the application and documents, and if no convictions are found, the Non-Conviction Certificate (NCC) will be issued.
Discuss Your NCC Needs with Us
Contact Regulatory Solutions India (RSI) today to discuss your NCC needs. With our expertise, we can help you obtain the Non-Conviction Certificate efficiently, giving your medical device business a competitive edge. Additionally, we specialize in regulatory consulting services for medical devices, IVDs, and cosmetics.
We are eager to partner with you. Drop us a message here with your specific requirements, and we will take it from there.