The Indian healthcare sector is rapidly expanding and is expected to reach $280 billion by 2025. The medical device market in India is one of the top twenty in the world. It currently has an estimated value of $11 billion, and by 2025, it is anticipated to grow astoundingly. India does not manufacture many medical devices on its own and still imports roughly 70% of its medical devices.
Medical device manufacturing and monitoring are highly regulated activities. The Central Drugs Standard Control Organization (CDSCO) introduced the Indian Medical Device Rules, 2017, to regulate medical devices, which were previously governed by the Drugs and Cosmetics Act of 1940.
To ensure full compliance in the rapidly evolving regulatory landscape, it’s essential to not only understand CDSCO registration but also how to acquire a medical device manufacturing license in India. This additional step enables manufacturers to contribute directly to India’s burgeoning healthcare sector.
What is CDSCO?
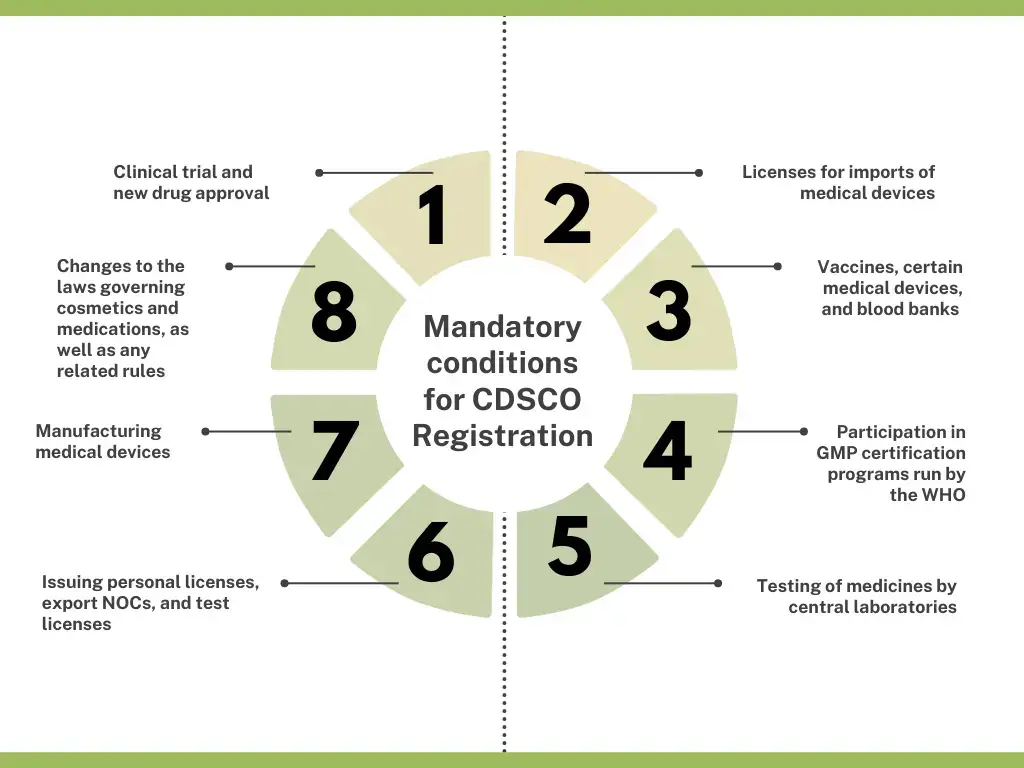
CDSCO registration is necessary, in the case of:
- Clinical trial and new drug approval
- Licenses for imports of medical devices
- Manufacturing medical devices
- Vaccines, certain medical devices, and blood banks
- Changes to the laws governing cosmetics and medications, as well as any related rules
- Participation in GMP certification programs run by the WHO
- Issuing personal licenses, export NOCs, and test licenses
The new CDSCO Medical Device Registration in India
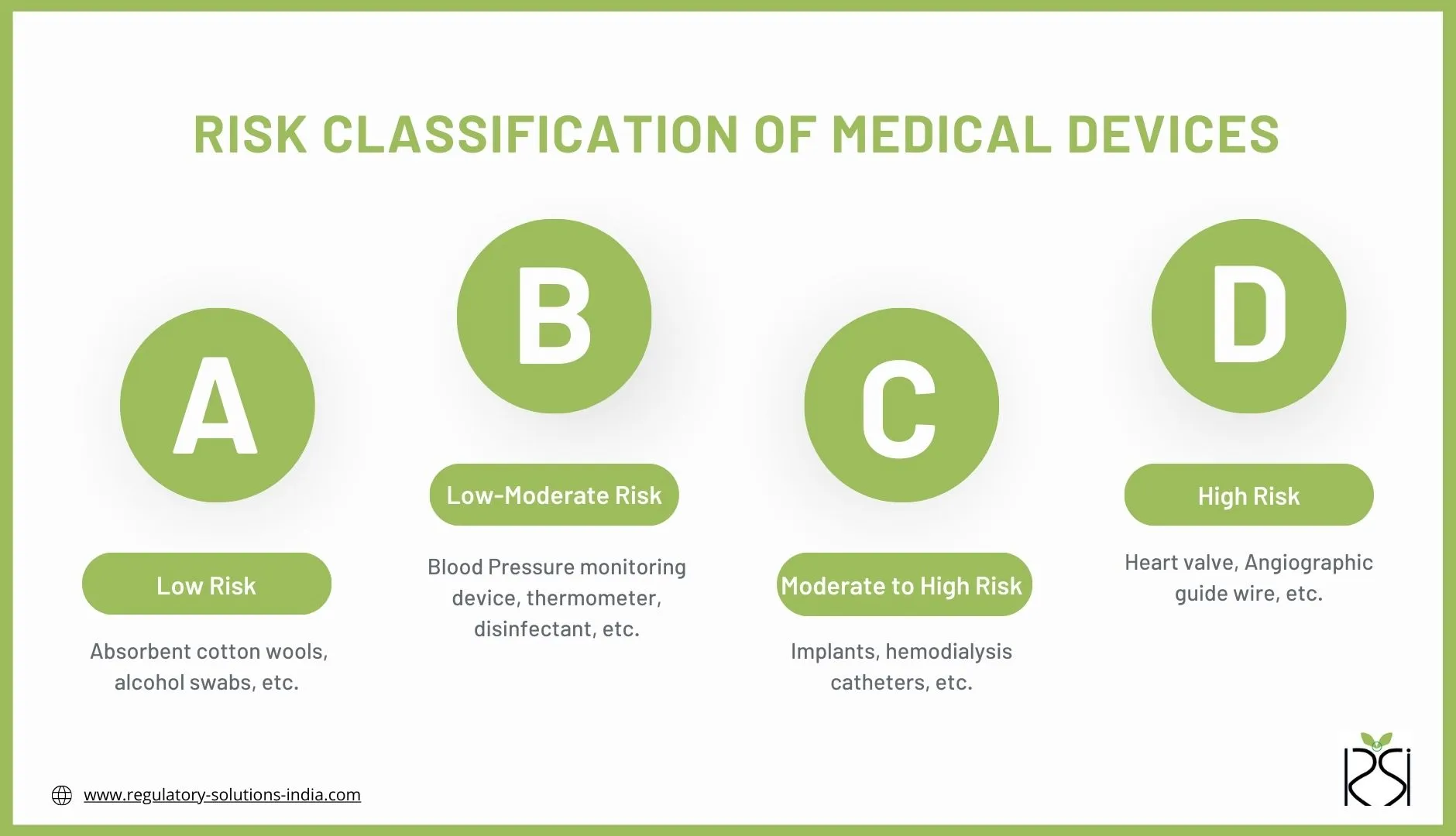
Risk classification of Medical Devices
Medical devices are generally classified according to their risks; the actual risk-based classification of the medical device is determined by its intended use and purpose.
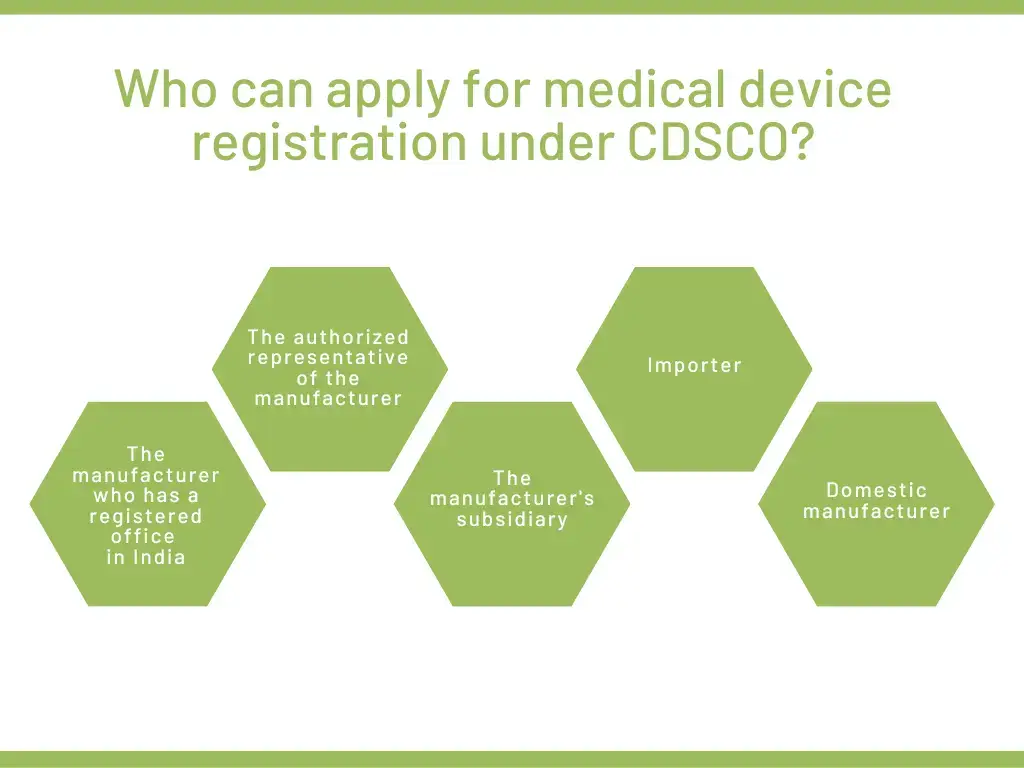
Who can apply for medical device registration under CDSCO?
What are the requirements for granting a Medical Device Import License?
Medical devices entering India must comply with the CDSCO’s Indian Medical Device Regulation. CDSCO has established a comprehensive procedure for issuing licenses for medical devices imported into the country. This procedure is followed when medical devices are imported into India from other countries. They must, however, be classified according to the CDSCO notified devices list. The Central Drugs Standard Control Organization has prescribed the following forms for importers to obtain a medical device import license.
For existing products
In general, for an importer who wishes to obtain a medical device import license for pre-existing goods, CDSCO has prescribed “Application Form: MD -14” and the authorization to import is received in “Form: MD -15”.
These forms are for all risk categories from A to D. (Some medical devices may also require a Compulsory Registration prior to obtaining the Import License).
New devices
In the event that an importer wishes to obtain an import license for new medical devices for which predicates do not exist, CDSCO has prescribed “Application Form: MD -26” and the approval is received vide “Permit Form: MD -27”
These forms are for all medical devices that fall under the categories from A to D.
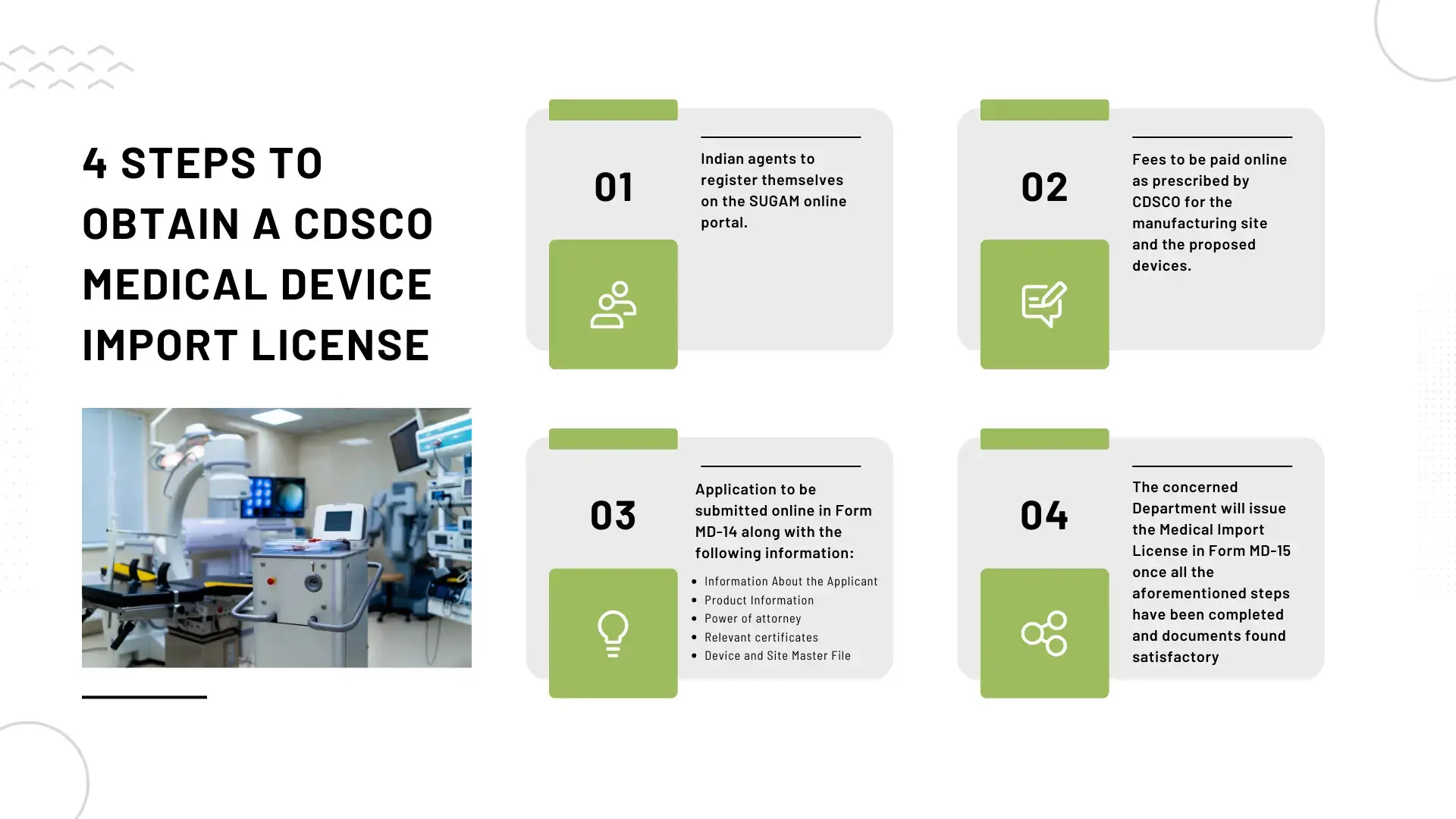
How to obtain a CDSCO medical device import license?
The Importer or Manufacturer or their agent in India must submit an application for the CDSCO Medical Device Import License about the facilities & devices manufactured by the Manufacturer and intended for import into India as per following steps:
Step 1: Indian agents register themselves on the SUGAM online portal.
Step 2: Fees are to be paid online as prescribed by CDSCO for the manufacturing site and the proposed devices.
Step 3: Application to be submitted online in Form MD-14 along with the following information:
- Information About the Applicant
- Product Information
- Power of attorney
- Relevant certificates
- Device and Site Master File
Step 4: The concerned Department will issue the Medical Import License in Form MD-15 once all the aforementioned steps have been completed and documents found satisfactory.
Additionally, it’s important to note that the Medical Device Import License in India is initially valid for 5 years and requires renewal every 5 years from its date of issuance.
How we’ll help you get a CDSCO Medical Device import license?
FAQ's
How do I register my medical device on CDSCO?
The complete registration process for medical devices is through the online portal of CDSCO i.e. “SUGAM”. Depending on the regulatory pathway identified for a device, the application needs to be submitted accordingly.
What are the medical devices requiring registration by CDSCO?
In line with the new definition adopted by CDSCO vide notification dtd.11th Feb 20, all devices which fall under the following definition require registration from CDSCO:
All devices including an instrument, apparatus, appliance, implant, material, or another article, whether used alone or in combination, including software or an accessory, intended by its manufacturer to be used especially for human beings or animals which does not achieve the primary intended action in or on human body or animals by any pharmacological or immunological or metabolic means, but which may assist in its intended use by such means for one or more of the specific purposes of:
(i) diagnosis, prevention, monitoring, treatment, or alleviation of any disease or disorder;
(ii) diagnosis, monitoring, treatment, alleviation, or assistance for, any injury or disability;
(iii) investigation, replacement or modification, or support of the anatomy or of a physiological
process;
(iv) supporting or sustaining life;
(v) disinfection of medical devices; and
(vi) control of conception.