Introduction :
As per the Medical Device Rule, 2017, the medical device import license in India needs to be renewed every five years from the date of issuance. The manufacturers/Importers who obtained their medical device import license in 2018 during the initial phase of the Medical Device Rule’s 2017 implementation are scheduled to expire in 2023. In this blog, we will take you on a journey through the CDSCO medical device import license renewal process and provide you with the guidance you need.
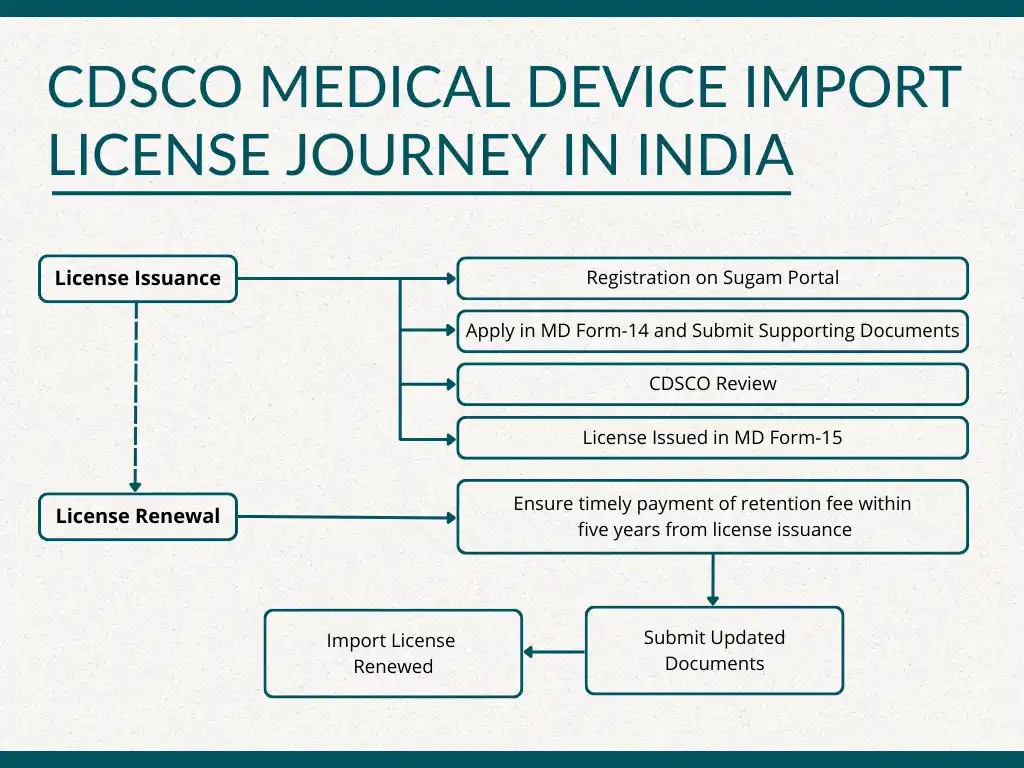
How to Obtain a CDSCO Medical Device Import License in India?
Acquiring a CDSCO medical device import license in India involves a meticulous and time-consuming process that entails comprehensive documentation. Follow these essential steps to obtain the import license for your medical device:
Step 1: Register yourself on Sugam Portal.
Step 2: Pay the fee prescribed by CDSCO.
Step 3: Submit online application in Form MD-14 with the required documents.
Step 4: The Concerned Authority will review your application and supporting documents. If found satisfactory, they will issue an import license in MD Form-15.
For more information on the process of registration and obtaining a medical device import license in India, read our blog on “The Complete Guide to CDSCO Medical Device Registration.“
Understanding the Validity and Renewal Process:
According to the Medical Device Rule of 2017, the import license remains valid perpetually unless cancelled or voluntarily surrendered. To ensure its ongoing validity, the license holder must pay the retention fee and submit updated documents to the Central Drugs Standard Control Organization (CDSCO), the government agency responsible for regulating medical devices in India.
The license holder is required to pay this fee for each overseas manufacturing site and each licensed medical device before every five years from the date of issuance. If payment is not made within the specified timeframe, there is an additional 90 days to pay, albeit with a late fee of two percent per month. Failure to pay the retention fee within the given period results in the license being considered cancelled.
Renewing the medical device import license requires two steps:
1. Pay the retention fee.
2. Submit the updated documents.
Documents Required for Medical Device Import License Renewal:
The following documents are required for CDSCO medical device import license renewal:
1. Covering letter with purpose of application.
2. Details of Base license and endorsement license, if any.
3. List of products to be removed (if applicable) with reasons.
4. Requisite Fees breakup basis the product class and manufacturing sites.
5. An undertaking by the manufacturer, that there is no change in Device master file (DMF) and Plant master file (PMF).
6. Post marketing surveillance data (Vigilance reporting) during last 5 yrs.
7. Valid Free Sale Certificate/Marketing Authorization from the country of origin (if any) and from any of the following countries viz USA, EU, UK, Canada, Japan, Australia.
8. Sales data of each device in India during last 5 yrs.
9. Valid copies of Quality Certificate ISO 13485 and applicable EC design certificates.
10. An undertaking by the manufacturer and authorized agent, stating that they are agreed for further retention of the Import License.
11. Details of Post Approval Change Application, If Any.
Import License Retention Fee:
The retention fees for the import license vary depending on the type and class of the medical device. Here’s a breakdown of the retention fees:
| S.R. No. | Types of Devices | Retention Fee |
|---|---|---|
| 1 | One overseas site, Manufacturing Class A medical device (excluding in vitro diagnostic) | $1000 |
| 2 | One overseas site, Manufacturing Class B medical device (excluding in vitro diagnostic) | $2000 |
| 3 | One overseas site, Manufacturing Class C or Class D medical device (excluding in vitro diagnostic) | $3000 |
| 4 | Each distinct Class A medical device (excluding in vitro diagnostic) | $50 |
| 5 | Each distinct Class B medical device (excluding in vitro diagnostic) | $1000 |
| 6 | Each distinct Class C or Class D medical device (excluding in vitro diagnostic) | $1500 |
| 7 | One overseas site, Manufacturing Class A or Class B in vitro diagnostic medical device | $1000 |
| 8 | One overseas site, Manufacturing Class C or Class D medical device (excluding in vitro diagnostic) | $3000 |
| 9 | Each distinct in vitro diagnostic medical device of Class A or Class B | $10 |
| 10 | Each distinct in vitro diagnostic medical device of Class C or Class D | $500 |
Conclusion:
Renewing your medical device import license is crucial to comply with the requirements of the Medical Device Rule of 2017. The renewal process involves paying the retention fee and submitting the updated documents. Regulatory Solutions India can assist you with the renewal process and ensure that your license remains valid.
Contact us today to learn more about our services and discover how we can support you in meeting your regulatory requirements.
FAQ
Q-1 What is MD Form-14?
MD Form-14 is an online application form for obtaining a medical device import license in India.
Q-2 In which form is a medical device import license issued in India?
The medical device import license in India is issued in the form of MD 15. The license is valid perpetually as long as the applicant pays the retention fee.
Q-3 What is the validity of an import license issued for MD 15 in India?
The import license issued for MD-15 in India is valid indefinitely as long as the retention fees are paid, and updated documents are submitted every five years.