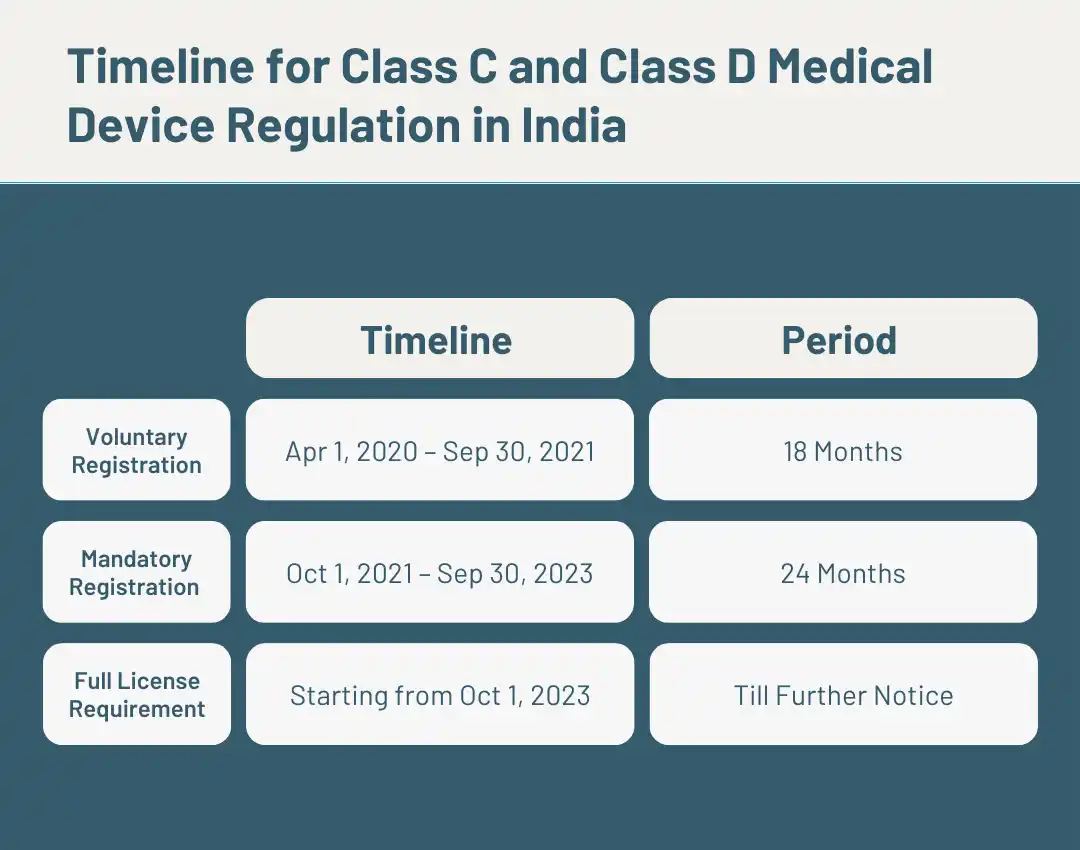
On October 12, 2023, the CDSCO released a circular, extending the deadline by six months for manufacturers and importers dealing with non-notified Class C and Class D medical devices. Originally slated for mandatory licensing from October 1, 2023, this extension aims to smoothen the transition. In this article, we delve into the intricacies of the CDSCO circular and guide you through the approval procedures for Class C and Class D medical devices.
CDSCO Addresses the Industry Concern
The circular grants manufacturers and importers of class C and D devices a six-month grace period to continue operations seamlessly. The shift to a fully licensed regime has triggered concern in the medical industry, prompting fears of a potential shortage of medical devices. Various stakeholders voiced apprehensions about the disruption. Acknowledging these concerns, the CDSCO extended the mandatory licensing timeline, allowing those who applied for licenses on or before September 30, 2023, to import or manufacture devices for an additional six months from 01st Oct 23.
Overview of the Registration and Licensing Process
The registration process for medical devices in India is intricate and demands substantial documentation. The CDSCO serves as the central authority, responsible for issuing import and manufacturing licenses for all medical devices. Here’s a concise breakdown:
Import Licensing Procedure for Class C and D Medical Devices
– Register on the Sugam portal.
– Pay the prescribed fee.
– Submit an online application on Form-14 with required documents.
– Upon review and satisfaction, CDSCO issues an import license in Form-15.
Procedure for Issuing a Manufacturing License for Class C and D Medical Devices
– Register on the Sugam portal.
– Pay the prescribed fee.
– Submit an online application in Form MD-7 (or MD-8 for loan License) with the necessary documentation.
– Undergo an audit of manufacturing facility by Central Licensing Authority.
– Upon compliance and satisfactory inspection, receive the manufacturing authorization in Form 9 (or MD-10 for loan License).
Conclusion:
The CDSCO’s extension of the licensing period addresses the medical device industry’s concerns, aiming to prevent disruptions in the manufacturing and import of medical devices. This initiative ensures a smoother transition to the new approval regime.
How RSI Supports Class C and D Medical Device Licensing:
If you have still not submitted your application for Class C & D devices, we could assist you with the same. With over a decade of experience in the medical device regulatory domain, RSI has successfully registered 450+ medical devices across all categories and fostered success stories for clients from over 15 countries.
Explore RSI’s spectrum of services:
Ready to navigate the regulatory landscape?
Contact us today to script your success story with RSI.
FAQ
1. Who is eligible for the 6-month extension provided by CDSCO?
The extension applies to manufacturers and importers who applied for licenses before September 30, 2023, allowing them to continue importing or manufacturing devices for an additional six months from 1st Oct 23.
2. What is Form-14 used for in the medical device licensing process?
Form-14 is an essential legal application form for obtaining an import license for medical devices in India. It requires submitting necessary documents alongside the application on the Sugam portal.
3. What is Form-15 in the import licensing procedure?
Form-15 is issued by the CDSCO upon a satisfactory review of Form-14, allowing the importation of medical devices into India once approved.
4. What is Form-7 in the medical device licensing process?
Form-7 is an essential application form used to obtain a manufacturing license for medical devices in India.
5. What is the significance of Form-9 in the medical device manufacturing process?
Form-9 is the permission granted upon satisfactory inspection and compliance, signifying the authorization for manufacturing Class C & D medical devices in India.