A CDSCO medical device manufacturing license is required for companies that manufacture medical devices in India. Obtaining a manufacturing license for medical devices in India is subject to a plethora of conditions and paperwork under the CDSCO.
The license is issued by the CDSCO and is necessary to ensure that the medical devices being manufactured meet certain quality and safety standards. The manufacturer must also ensure that their products are in compliance with the regulations laid down by the CDSCO before they can be marketed in the country.
A CDSCO medical device manufacturing license is required for companies that manufacture medical devices in India. Obtaining a manufacturing license for medical devices in India is subject to a plethora of conditions and paperwork under the CDSCO.
The license is issued by the CDSCO and is necessary to ensure that the medical devices being manufactured meet certain quality and safety standards. The manufacturer must also ensure that their products are in compliance with the regulations laid down by the CDSCO before they can be marketed in the country.
Documentation required to apply for a medical device manufacturing license
To apply for a Medical Devices Manufacturing License, an applicant must provide extensive documentation to demonstrate compliance with regulatory requirements. This includes information about the premises, the company’s legal structure, the identities and qualifications of the staff, technical information about the manufacturing process, and various certifications and approvals from relevant government agencies. The documentation must be self-attested, and applicants must pay a license fee and any additional charges.
Forms to be filled in order to receive a manufacturing license for medical devices with different classification
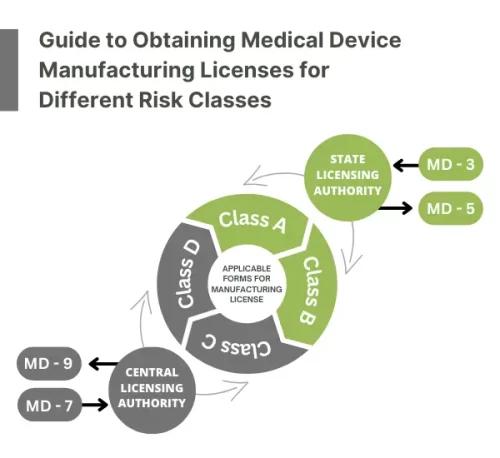
For Medical Devices with Risk Class A & B
The manufacturing license for Class A and Class B medical device sales and distribution is regulated by the State Licensing Authority. Form MD-3 must be completed in order to obtain a license, which is issued on Form 5.
For Medical Devices with Risk Class C & D
The manufacturer must submit an online application using Form MD-7 to the Central Licensing Authority of the Ministry of Health and Family Welfare of the Central Government to obtain a medical device manufacturing license for the continued sale and distribution of Class C and Class D medical devices. The license is issued in form MD-9 once the application is approved.
Permission for Loan License to Manufacture Medical Devices
1. For Class A – B Medical Devices, apply in Form MD-4 and the license is granted in form MD-6.
2. For Class C – D Medical Devices, apply in Form MD-8 and the license is granted in form MD-10.
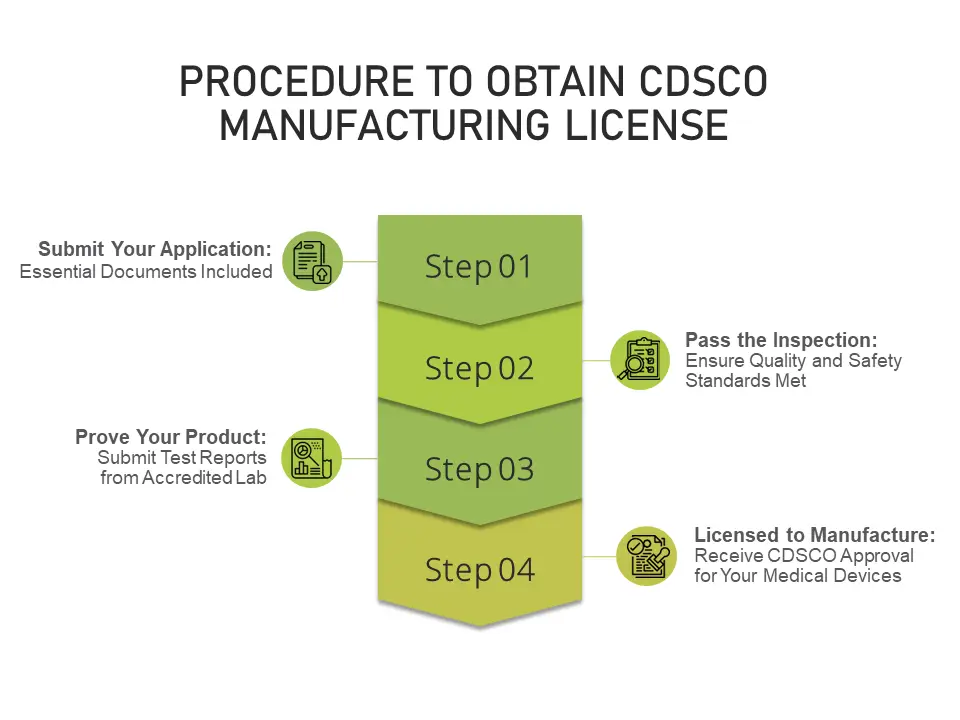
Procedure to obtain a medical device manufacturing license
Obtaining a medical device manufacturing license in India typically entails the following steps:
Step 3: Audit of applicant premises by a notified body.
Step 4: Upon receipt of the audit report, it is examined and if found satisfactory, it will be forwarded for the next step.
Step 5: Scrutiny of the details of the products applied to ensure compliance with norms.
Step 6: If all the prescribed conditions are complied with, the manufacturing license is granted.
Role of ISO 13485 in obtaining manufacturing license for medical devices in India
What is ISO 13485?
The ISO 13485 standard is a set of requirements that govern the quality management systems (QMS) of organizations that design and manufacture medical devices. The ISO 13485 standard is focused on QMS effectiveness and meeting regulatory and customer requirements. The current ISO 13485 edition was published on March 1, 2016 and is based on ISO 9001:2008 with a greater focus on risk management and additional documentation requirements.
While ISO 13485 is not a mandatory requirement for manufacturing medical devices in India, it is highly recommended as it demonstrates a commitment to quality and safety and can help companies to meet the regulatory requirements for medical device manufacturing. Many countries, including the United States and European Union, require that medical device manufacturers have an ISO 13485-compliant QMS in place. So, if a company or a manufacturer wants to export its products to other countries, it should comply with ISO 13485.
Additionally, CDSCO also recognizes ISO 13485 as a standard for QMS for Medical Device manufacturers. Hence, it’s always recommended to comply with the standard to have a robust QMS system in place and increase the chances of getting a license from CDSCO.
About Regulatory Solutions India
Regulatory Solutions India (RSI) was founded in 2011 and since then has registered, in India, over 400+ products including Medical Devices, IVD, and Cosmetics across 25+ categories viz Stents, Catheters, Intraocular lenses, Orthopedic Implants, Ablation devices, Surgical dressings, Hypodermic Syringe/Needle, etc. for clients from 15+ countries. RSI offers a unique blend of technical, strategic, and project management support to all its clients backed by its rich industry experience. We help companies register their products with the central licensing authority in India i.e. CDSCO while offering expert insights into the CDSCO medical device registration process and the CDSCO medical device import license process to navigate the regulatory landscape effectively.