Introduction:
On April 12, 2023, the Central Drugs Standard Control Organization (CDSCO) of India released a circular reminding Indian manufacturers and importers that all Class C and Class D non-notified medical devices, currently under mandatory registration, will require a full license starting October 1, 2023. This blog will provide you with an overview of the new licensing regime and assist you in transitioning to the new license requirements.
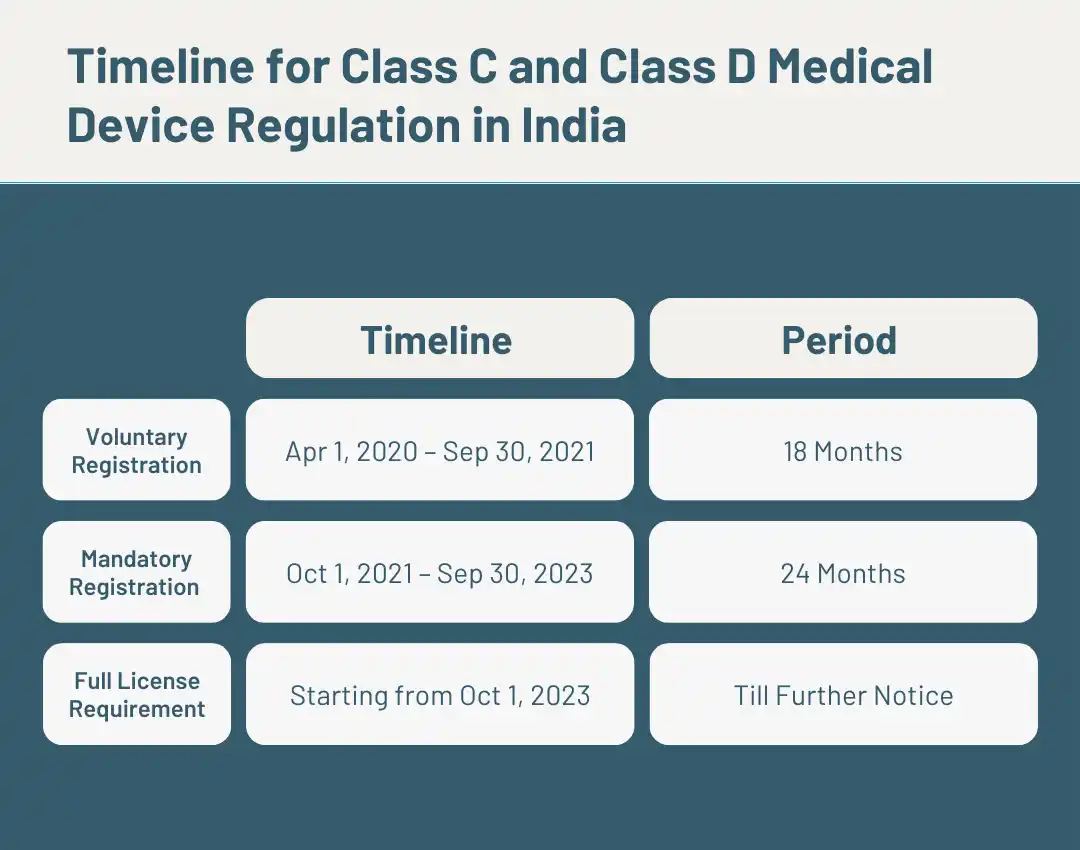
Full License Requirement for Class C and Class D non-notified medical device
It is important first to understand what is meant by a non-notified medical device. These are medical devices which have fallen under the ambit of the licensing regime after the adoption of the new enlarged definition for Medical Devices by Ministry of Health & Family Welfare in Feb 2020. (Not sure whether your device is non-notified or not? We can help)
This full license requirement deadline is the final step for transiting the Class C & Class D non-notified medical devices to the Licensing regime. Currently, all class C and D non-notified medical devices are exempt from Manufacturing/Import licence and can be manufactured/ imported into India basis their mandatory registration with CDSCO. From October 1, 2023, for such devices, it will compulsory to have a license if you want to introduce your medical device in India.
For medical device importers:
Getting a CDSCO medical device import license in India can take up to six months post the submission of application. The preparation for the application submission is also a time-consuming process and can easily take 1-3 months depending on the level of preparedness of an organization. Hence, it is important that all Class C and Class D non-medical device importers apply for the import license at the earliest to avoid any business disruption post 1st Oct 23.
For medical device manufacturers:
Similarly, obtaining a medical device manufacturing license in India is a time consuming process involving not only the extensive documentation but also an inspection by a Medical Device Officers of the Central Licensing Authority (CLA) within 60 days of application submission. Hence, if you have not started the application process, there is an urgency to do so now to maintain business continuity.
Conclusion:
The transition from mandatory registration to manufacturing/import license for Class C and D non-notified medical devices is the final step towards the full licensing regime for such devices. Manufacturers/importers need to be aware of the new regulation and take proactive steps to obtain a manufacturing/ import license. Given that CDSCO can take up to 6 months to issue a license there is an urgent need for impacted importers/manufacturers to start their application process immediately, if not done till now. Timely compliance will not only avoid disruption in the supply chain but also ensure continued access to these medical devices for patients.
This full license requirement deadline is the final step for transiting the Class C & Class D non-notified medical devices to the Licensing regime. Currently, all class C and D non-notified medical devices are exempt from Manufacturing/Import licence and can be manufactured/ imported into India basis their mandatory registration with CDSCO. From October 1, 2023, for such devices, it will compulsory to have a license if you want to introduce your medical device in India.
Why Choose “Regulatory Solutions India” for your regulatory compliance needs?
When it comes to your regulatory compliance needs, Regulatory Solutions India (RSI) stands out as a trusted and reliable choice. With over 12 years of experience and a track record of success since its establishment in 2011, RSI has effectively registered over 400 products in various categories, including medical devices, IVDs, and cosmetics. The satisfaction of our clients from 15+ countries is a testament to our commitment to excellence. With a team of seasoned regulatory professionals, we are well-equipped to handle all your compliance requirements. Our expertise extends to providing technical, strategic, and project management support to ensure your success. Whether you require assistance with CDSCO medical device registration, import licensing procedures, post-approval changes, or dossier preparation, RSI is here to help.
Don’t hesitate to contact us for all your regulatory compliance needs.
FAQ
What are Class C and Class D medical devices in India?
According to the Medical Device Rule of 2017, medical devices in India are classified into four categories: Class A, Class B, Class C, and Class D, based on their intended use, level of risk, and other factors. Class C and Class D medical devices are considered to have a moderate to high risk and are subject to stricter regulatory requirements compared to Class A and Class B medical devices.