Introduction :
The introduction of a medical device into the market is a complex process that starts with necessary approval from the licensing authority. Once a device has been approved, any changes made to it can potentially impact its performance, quality, safety, and effectiveness. To address this issue, the central government has included a provision in the sixth schedule of the Medical Device Rules (2017) that requires all post-approval changes to be communicated to the licensing authority.
What are Post-Approval Changes in Medical Devices?
Post-approval changes in medical devices refer to any modifications or alterations made to a device or to any of the manufacturing steps after an import license has been issued by CDSCO. These changes may include modifications to the device’s design, manufacturing processes, intended use, or indication for use. Any such changes have the potential to affect the device’s performance, quality, safety, and effectiveness. Therefore, it is crucial to manage these changes effectively to ensure that the device continues to meet regulatory requirements.
Types of Post-Approval Changes
Post-approval changes in medical devices can be classified into two categories: major changes and minor changes.
1. Major Changes: Major changes refer to any changes that have a significant impact on the device’s performance, quality, safety, or effectiveness. These changes include:
- Material of construction
- Design affecting the quality
- Intended use or indication for use
- Sterilization method
- Approved shelf life
- Name or address of licensee or manufacturer
- Labeling excluding font type/size, color, design
- Manufacturing processes, equipment, or testing affecting the quality
- Primary packaging material
If any major changes are made to the device, the manufacturer/Importer must notify the licensing authority and obtain approval before implementing the changes. The changes in medical devices must be approved or rejected by the central licensing authority within 45 days, otherwise the changes are deemed approved.
2. Minor Changes: Changes to the following are considered minor change:
- Design which does not affect quality in respect of its specifications. Indications for use, performance and stability of the medical device
- Manufacturing processes, equipment, or testing which does not affect quality of the device.
- Packaging specifications excluding primary packaging material.
If any minor changes are made to the device, the manufacturer must inform the licensing authority within 30 days of implementing the changes. However, prior approval from the licensing authority is not required for minor changes.
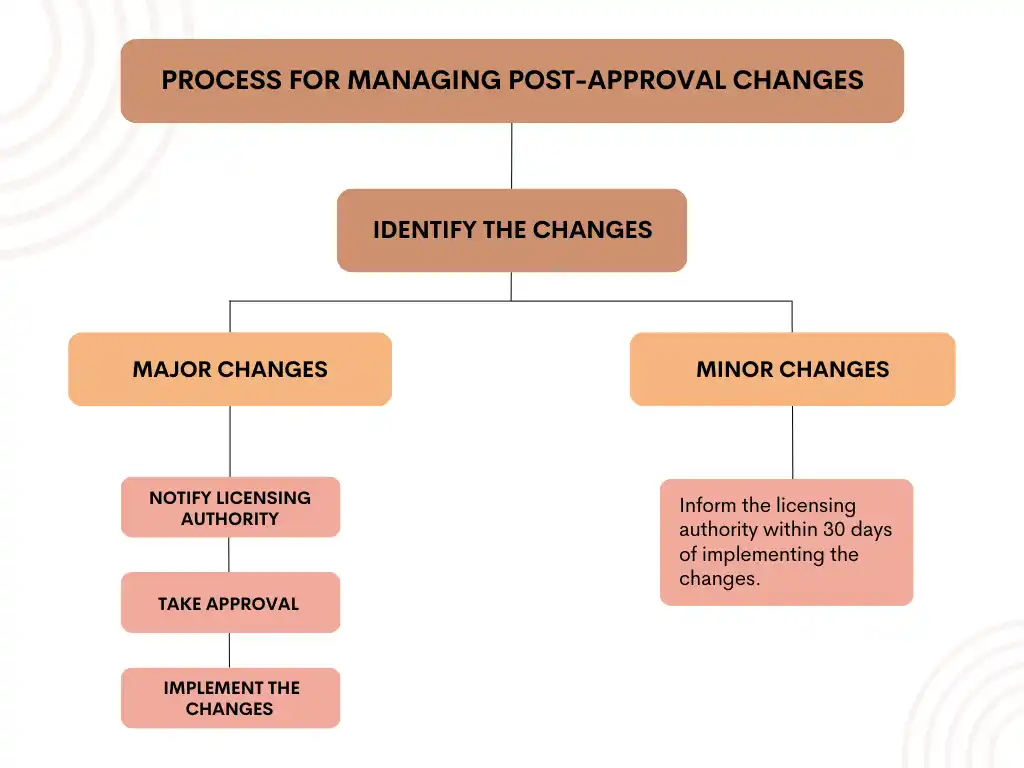
Process for Managing Post-Approval Changes
The process for managing post-approval changes in medical devices includes the following steps:
- Identification of Changes: The first step is to identify any changes that have been made to the device. This may involve conducting an impact assessment to determine whether the changes are major or minor.
- Notify and obtain approval from the Licensing Authority: If any major changes have been made to the device, the manufacturer/ importer must notify the licensing authority and obtain approval before implementing the changes. The notification must include details of the changes, such as the reason for the change and supporting documents.
Conclusion:
Post-approval changes in medical devices are an essential aspect of the development process. Manufacturers must be aware of the types of changes and the process for notifying the licensing authority about the changes. By ensuring compliance with regulations, manufacturers can make necessary changes to improve the device’s performance, quality, safety, and effectiveness while ensuring patient safety.
Regulatory Solutions India has a team of experienced regulatory consultants who can assist you in implementing post-approval changes and obtaining approval from the licensing authority. By working with our team, you can ensure compliance with regulations while making necessary changes to improve the device’s performance, quality, safety, and effectiveness.
Don’t hesitate to contact us to discuss your regulatory needs and how we can assist you.